Viral vectors and methods for producing and using the same
a technology of viral vectors and vectors, which is applied in the field of reagents and methods for producing viral vectors, can solve the problems of inability to complete viral replication or produce new virus particles, low yield, and inability to complete viral replication, so as to reduce or even eliminate contamination with adenovirus, improve aav production titers, and reduce the reliance on e1a+ packaging cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cell Culture
[0178]293 cells, C-7 cells (Amalfitano et al. (1999) Proc. Natl. Acad. Sci. U.S.A.
[0179]96:8861-8866), and GSD II patient fibroblasts were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum, 100 U penicillin per milliliter, and 100 μg streptomycin per milliliter at 37° C. in a 5% CO2-air atmosphere. C-7 cells were grown in the presence of hygromycin, 50 μg / ml. HeLa cells were maintained in minimum essential medium Eagle supplemented with 10% fetal bovine serum, 1 mm minimum essential medium sodium pyruvate, 0.1 mm minimum essential medium non-essential amino acids, 100 U penicillin per milliliter, and 100 μg streptomycin per milliliter at 37° C. in a 5% CO2-air atmosphere.
example 2
Transduction of Cultured Cells
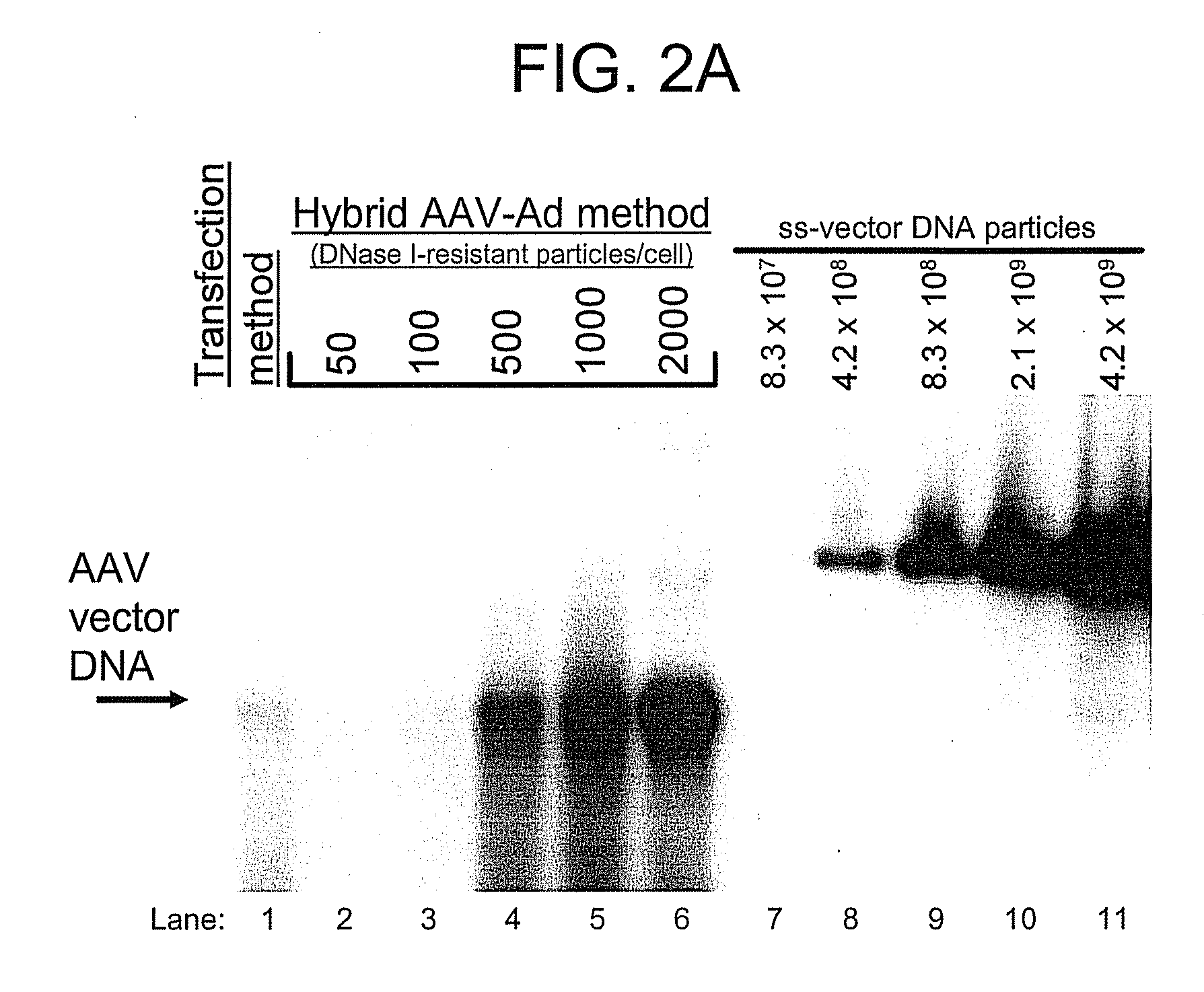
[0180]HeLa cells and GSD II fibroblasts were plated at 1×106 cells per 150 mm tissue culture dish. Cells were transduced the next day by adding a volume of the respective vector stock containing 1,000, 10,000, or 50,000 DNase I-resistant vector particles (as defined by Southern blot analysis) per cell. Cells were harvested five days later for hGAA measurement and Western blotting analysis.
example 3
Construction of an AAV Vector Plasmid Encoding hGAA
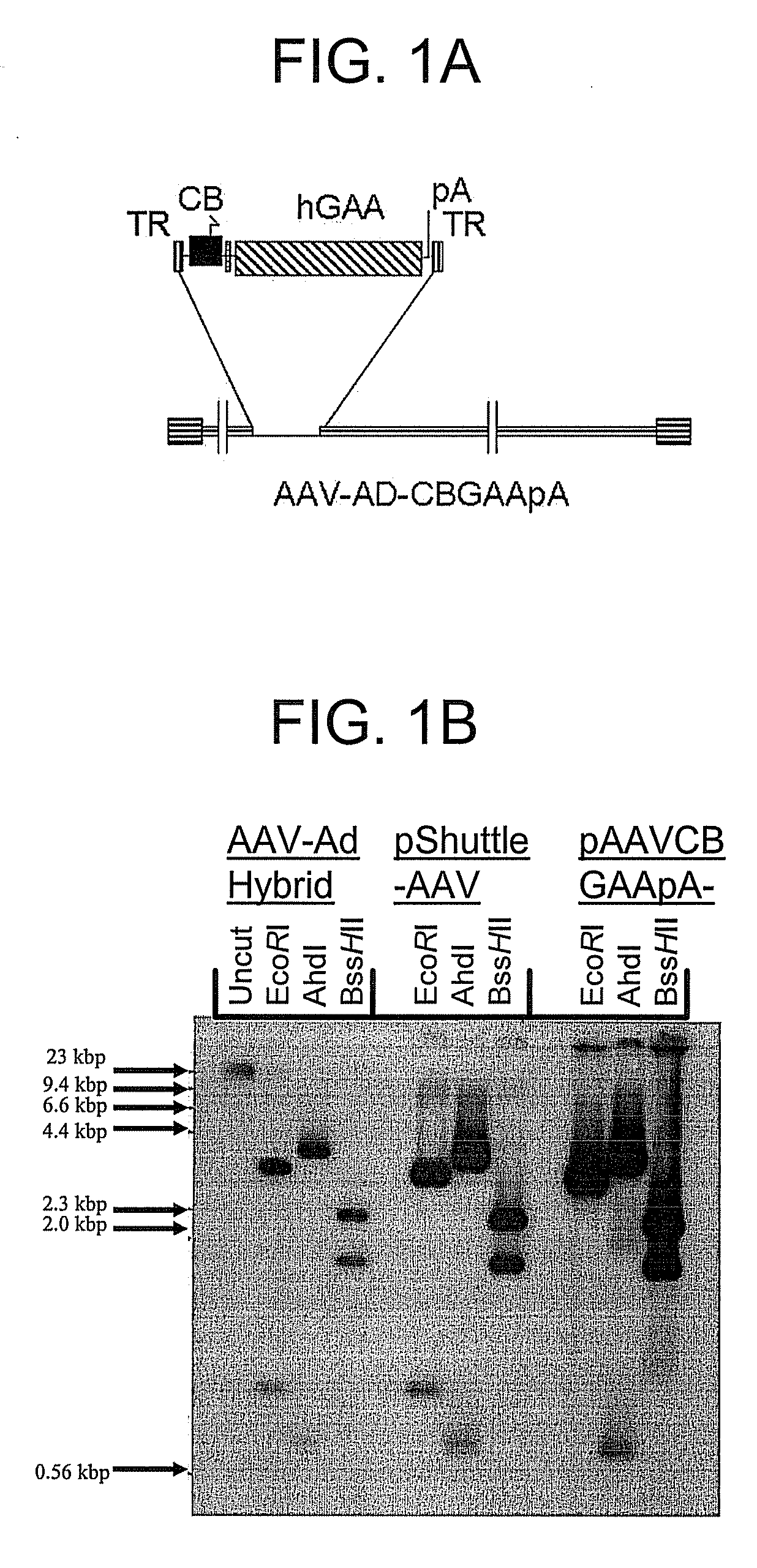
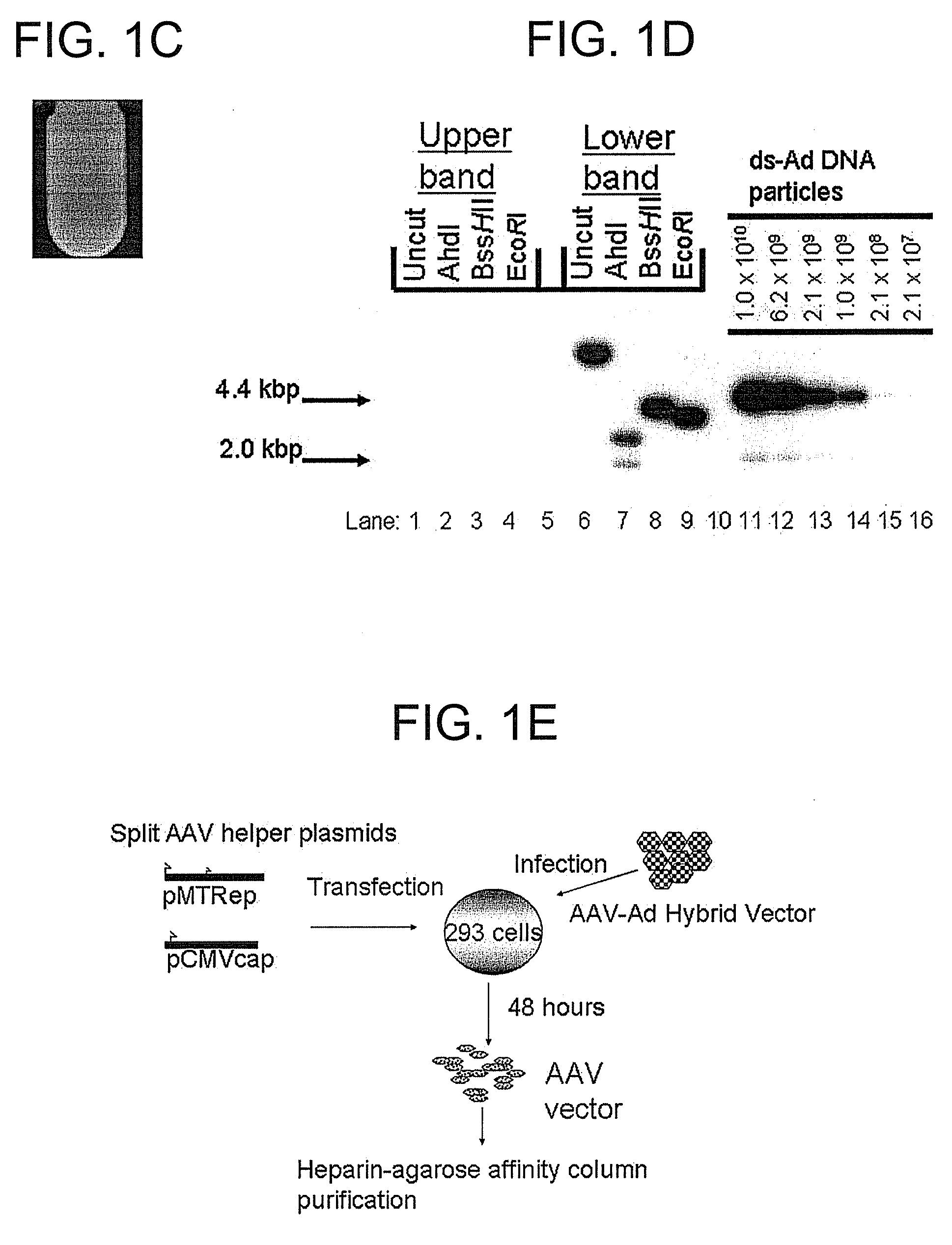
[0181]The hGAA cDNA was subcloned with the CMV promoter from pcDNA3-hGAA (Van Hove et al. (1996) Proc. Natl. Acad. Sci. U.S.A. 93:65-70) into an AAV vector plasmid to generate pAAV-CBGAApA. The vector sequences from pAAV-CBGAApA were isolated as a 4.4 kbp fragment from a partial BglII digest, and ligated with the calf intestinal alkaline phosphatase-dephosphorylated BglII site of pShuttle (He et al. (1998) Proc. Nat. Acad. Sci. U.S.A. 95:2509-2514).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com