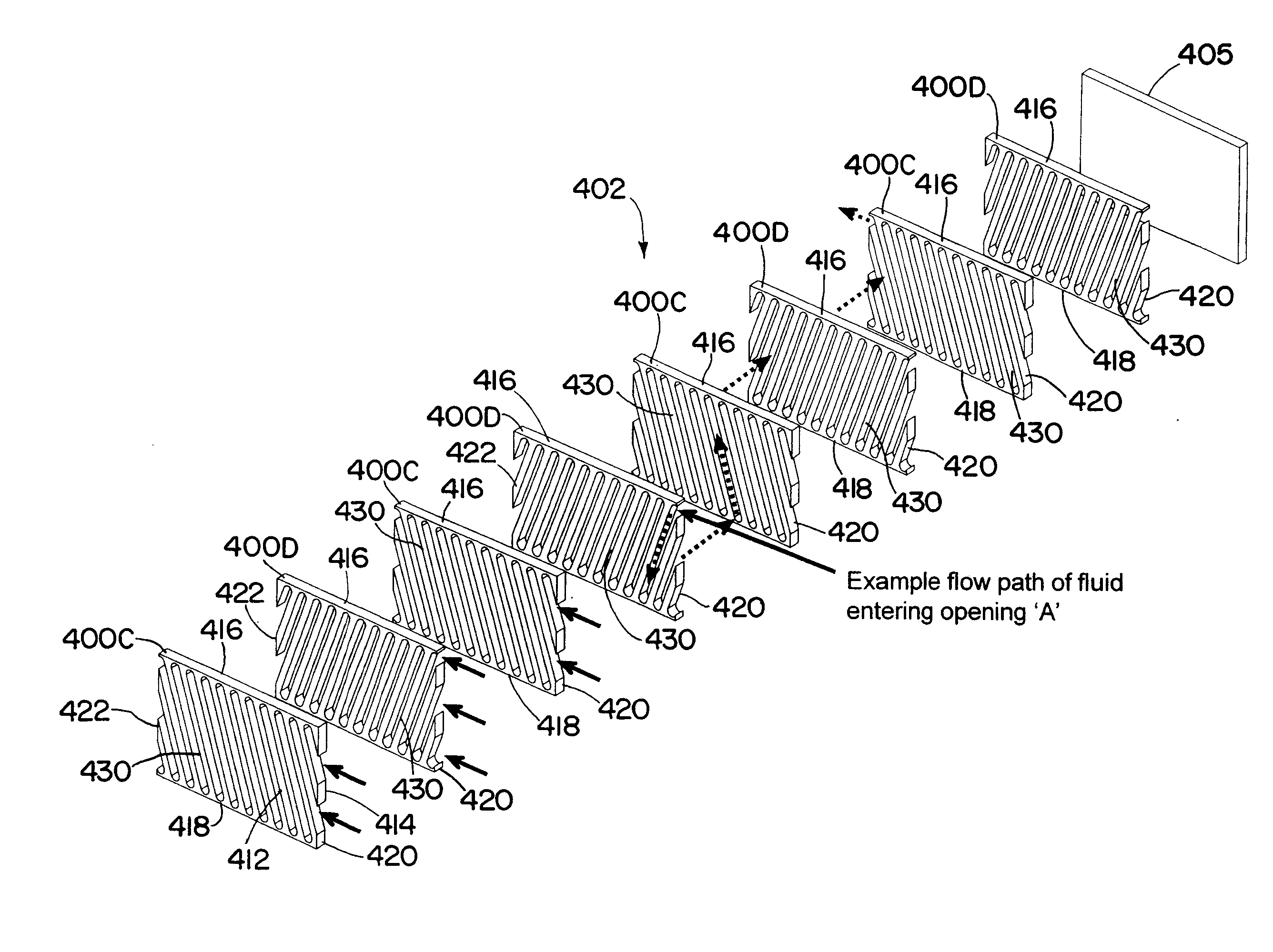
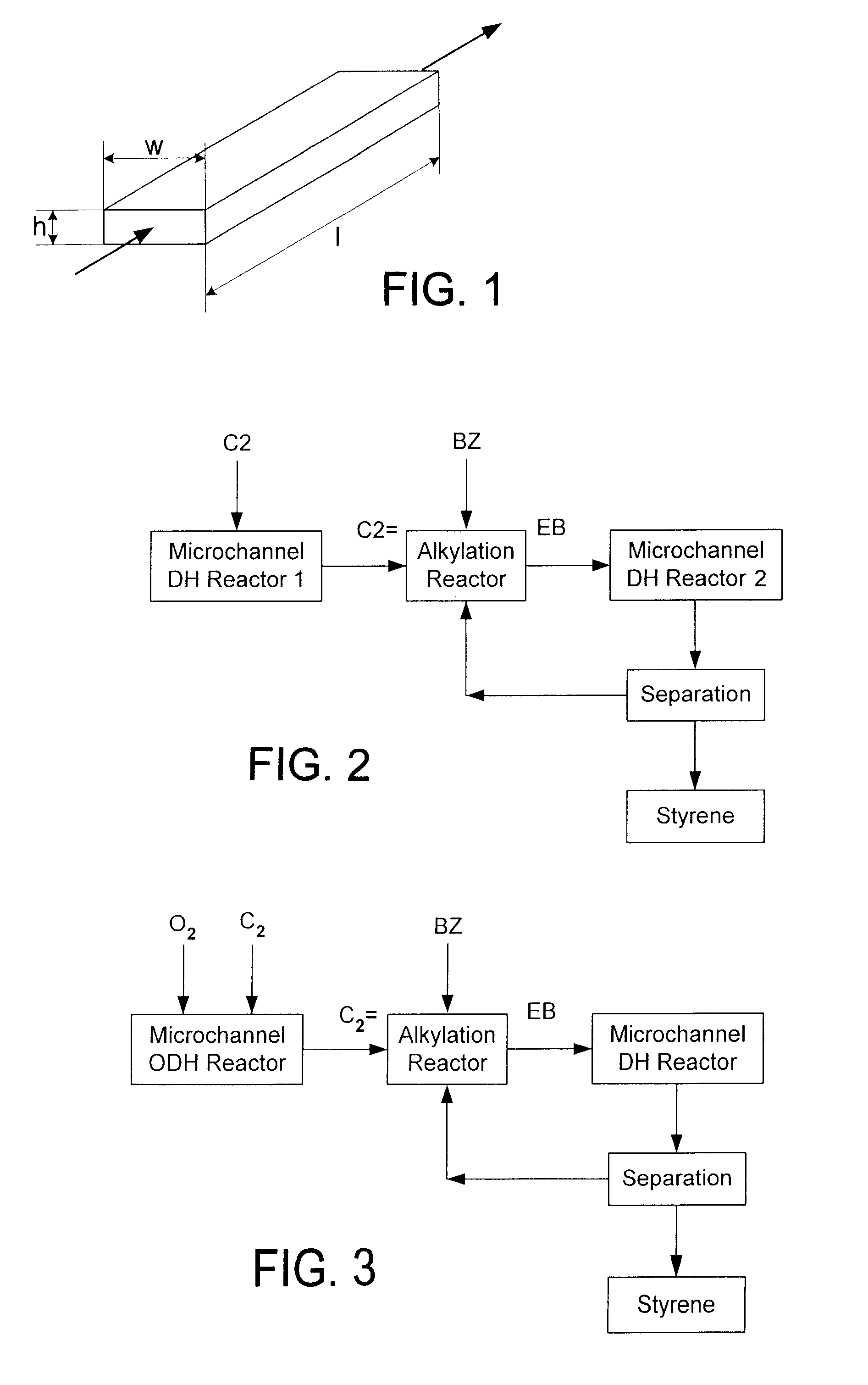
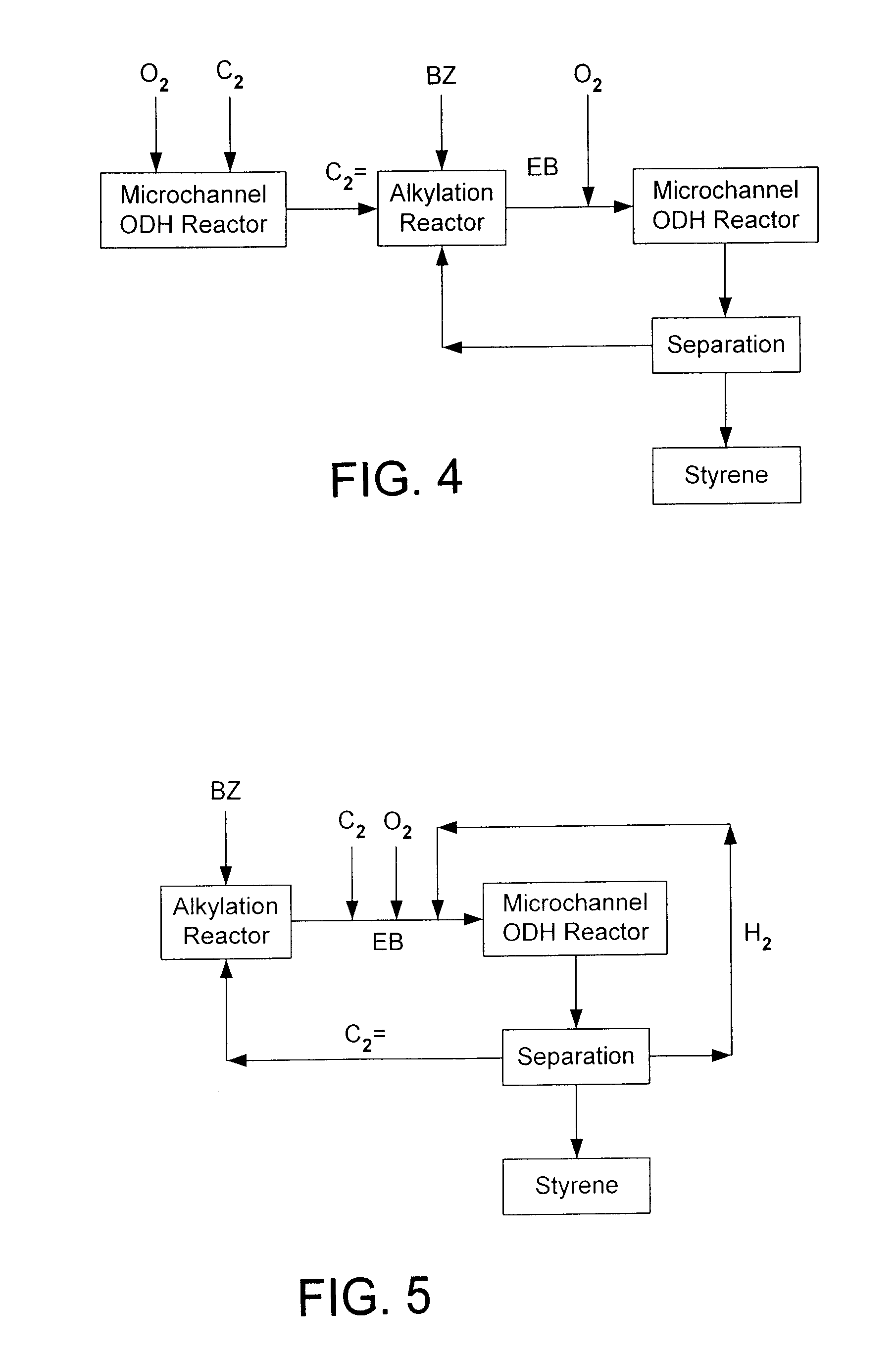
Process for making styrene using mircohannel process technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
0.7% K2O-15% MoO3 / SiO2—TiO2 catalyst is prepared by the sol-gel method. 20.0 g tetraethylorthosilicate and 27.29 g titanium isopropoxide are dissolved in 200 ml isopropyl alcohol solution with stirring. In another beaker, 2.93 g ammonium paramolybdate are dissolved in 13.65 g H2O and then 0.30 g 45% KOH solution are added. The aqueous solution is added dropwise to the alcohol solution (1 ml / min). After all of the aqueous solution is added, the resulting gel is stirred for additional 15 min. The gel is dried at 110° C. overnight and calcined at 550° C. for 5 hours. The catalyst is crushed and sieved to 60-100 mesh.
The catalyst (0.4 g) is loaded in a quartz tube reactor having a 0.2 inch O.D. (0.635 cm). The reactor volume is 0.3 ml. A feed gas composition containing 9.9% by volume ethylbenzene, 5% by volume O2 and 85.1% by volume N2 flows into the reactor. The feed gas flow rate is 180 ml / min. The contact time based on reactor volume is 0.1 second. The process operates for 3 hours wi...
example 2
0.7% K2O-18% V2O5 / SiO2—ZrO2 catalyst is prepared by the sol-gel method. 7.05 g vanadium (III) 2,4-pentanedionate are dissolved in 200 ml iso-butanol with stirring at 60° C. After cooling, 19.97 g zirconium n-butoxide are added at room temperature with stirring, followed by 15.0 g n-butoxysilane. In another beaker, 0.19 g 45% KOH solution are mixed with 6.63 g H2O. The aqueous solution is added dropwise to the alcohol solution (1 ml. / min). After all of the aqueous solution is added, the resulting mixture is stirred for an additional 15 min. The gel is then dried at 110° C. overnight and calcined at 550° C. for 5 hours. The catalyst is crushed and sieved to 60-100 mesh.
The catalyst (0.5 g) is loaded in the quartz tube reactor identified in Example 1. The feed gas composition contains 9.9% by volume ethylbenzene, 5% by volume O2 and 85.1% by volume N2. The contact time is 0.1 second. The process operates for 3 hours with no evidence of catalyst deactivation. The process is operated at ...
example 3
Mg0.99MoO3.99 catalyst is prepared by the sol-gel method. 16.00 g molybdenum (VI) oxide bis(2,4-pentanedionate) is dissolved in 200 ml methoxyethanol. 5.56 g magnesium ethoxide are then added with stirring.
Subsequently, 14.13 g 2.5 mol / L NH4OH solution are added dropwise to the mixture. The resulting gel is dried at 110° C. for 5 hours and then calcined at 550° C. for 12 hours. The catalyst is crushed and sieved to 60-100 mesh.
The catalyst (0.3 g) is loaded in the quartz tube reactor identified in Example 1. The feed gas composition contains 9.9% by volume ethylbenzene, 5% by volume O2 and 85.1% by volume N2. The contact time is 0.1 second. The process operates for 4 hours with no evidence of catalyst deactivation. The process is conducted at atmospheric pressure. The products are analyzed by GC. At 500° C., 29% ethylbenzene conversion and 88% styrene selectivity are achieved. The styrene yield is 26%. O2 conversion is 78%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Width | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com