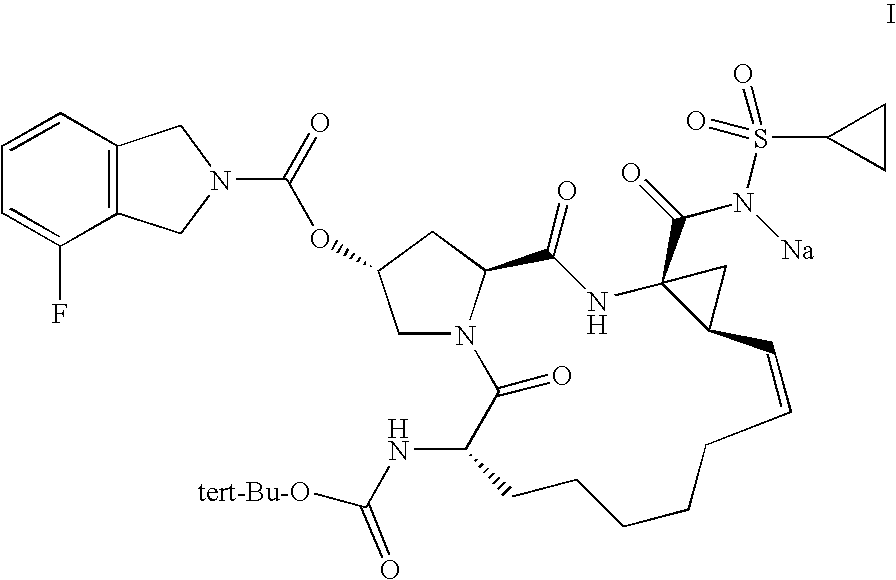
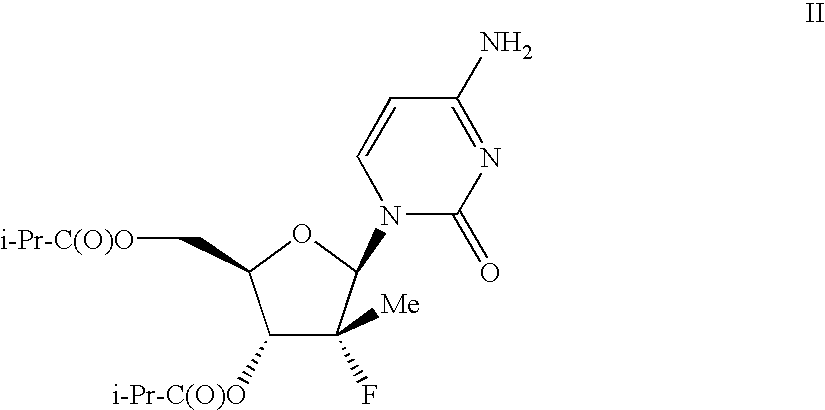
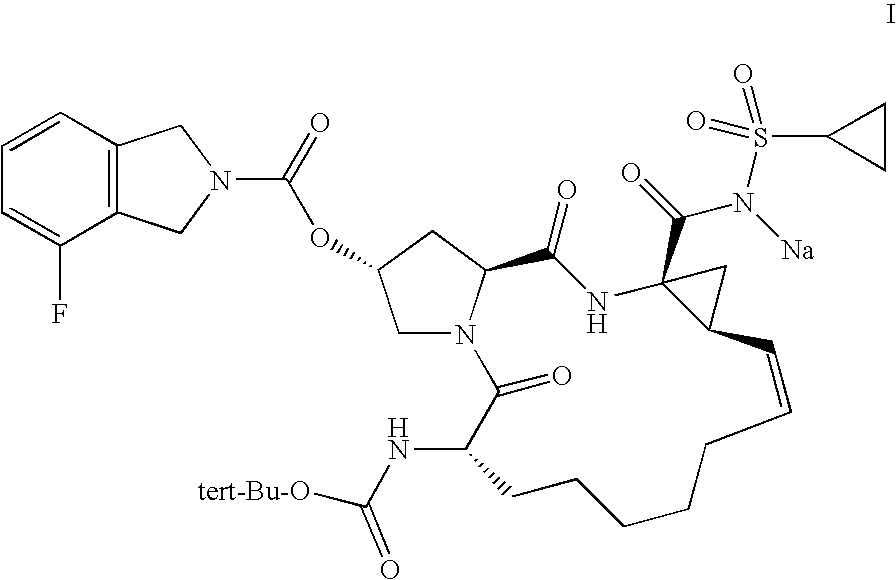
Method for improving pharmacokinetics
a pharmacokinetic and drug technology, applied in the field of improving pharmacokinetics, can solve the problems of ribavirin's significant toxicity, known to induce anemia, and difficult for many patients to tolerate the hematologic and constitutional toxicities of peg-ifn and rbv, and achieve the effect of increasing the bioavailability and or blood level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Pharmacokinetic Enhancement of R7227 in Healthy Adults
[0078]Subjects were screened for participation in this study within 21 days before dosing. The study enrolled 14 healthy volunteers (n=14 per group). The dosing schedule is illustrated below:
TABLE IStudy DayProcedure1234 to 1112R7227 dosing1xxxRitonavir Dosing2xxxPK collections24-h PK48-h PK48-h PK1R7227 100 mg single oral dose2Ritonavir 100 mg dose orally every 12 h.
[0079]R7227 formulation—R7227 was formulated into clear size 10 oval soft gelatin capsules for oral administration at a strength of 100 mg per capsule (anhydrous free acid equivalent). The capsule fill solution consists of R7227-001, polyethylene glycol PEG400 (Macrogol 400), and butylated hydroxytoluene (BHT). All excipients are compendial grade (NF or EP). Gelatin Type 195 (NF, EP) is used as the bulk gel mass for the capsule shell, with small amounts of sorbitol liquid 85 / 70 / 00 (NF, EP) and water (USP) used as plasticizers.
[0080]All study medications were with a m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass flow rate | aaaaa | aaaaa |
| Mass flow rate | aaaaa | aaaaa |
| Mass flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com