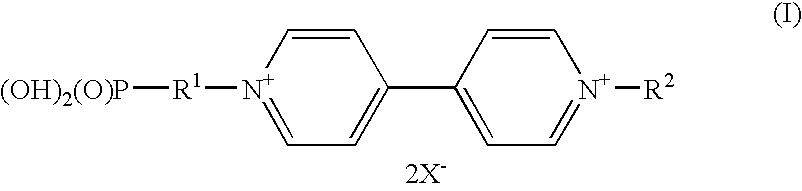
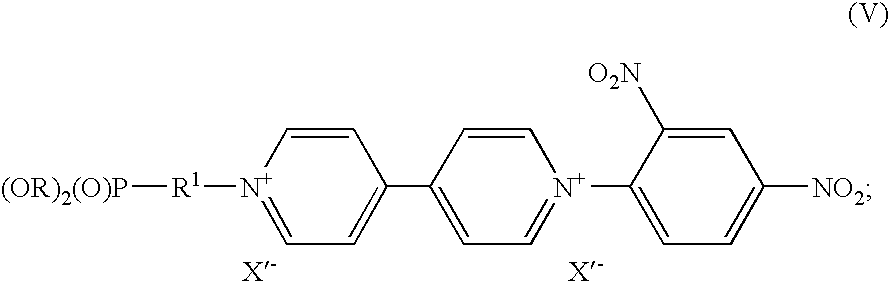
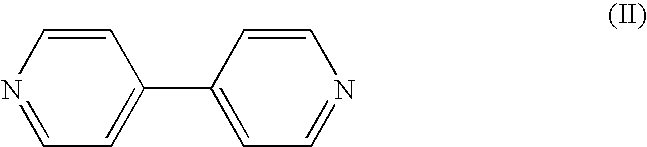
Electrochromic compounds
a technology of electrochromic devices and compounds, applied in the field of electrochromic devices, can solve problems such as preventing efficient decolouration of electrochromic devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Synthesis of 1-Phosphonoethyl-1′-(3-propylphenyl)-4,4′-bipyridinium bis-hexafluorophosphate (Compound 2)
[0068]A solution of ammonium hexafluorophosphate (5 g) in water (20 ml) was added to a cold solution of 1-phosphonoethyl-1′-(3-propylphenyl)-4,4′-bipyridinium dichloride (2 g) prepared in Example 1 in water (20 ml), A precipitate of 1 -phosphonoethyl-1′-(2,4,6-trimethylphenyl)-4,4′-bipyridinium bis-hexafluorophosphate (3 g) formed immediately and was filtered, washed with cold water and dried.
example 3
Synthesis of 1-Phosphonoethyl-1′-(2,4,6-trimethylphenyl)-4,4′-bipyridinium dichloride (Compound 3)
(i) Synthesis of 1-(Diethyl ethylphosphonate)-4,4′-bipyridinium bromide
[0069]4,4′-Bipyridine (100 g, 0.64 moles) and diethyl bromoethylphosphonate (157 g, 0.64 moles) in toluene (500 ml) in a 1 L round bottomed flask was refluxed until the solid precipitate of the monocation salt was formed. The precipitate was filtered hot. The filtrate was refluxed again and the process repeated until no more solid formed. A yield of 95 g of the monocation was obtained.
(ii) Synthesis of 1-Diethyl ethylphosphonate-1′-(2,4-dinitrophenyl)-4,4′-bipyridinium monobromide monochloride
[0070]1-Diethyl ethylphosphonate-4,4′-bipyridinium monobromide (50 g, 0.12 moles) was added to acetonitrile (400 ml) in a 1 L round-bottomed flask and refluxed for thirty minutes. The clear solution was decanted into a 1 L round-bottomed flask and 2,4-dinitrochlorobenzene (150 g, 0.74 moles) was added and refluxed for eighteen h...
example 4
Synthesis of 1-phosphonoethyl-1′-(2,4,6-trimethylphenyl)-4,4′-bipyridinium bis-hexafluorophosphate (Compound 4)
[0074]A solution of ammonium hexafluorophosphate (4.3 g) in water (20 ml) was added to a solution of 1-phosphonoethyl-1′-(2,4,6-trimethylphenyl)-4,4′-bipyridinium dichloride (2 g) prepared in Example 3 in water (20 ml). A precipitate of 1-phosphonoethyl-1′-(2,4,6-trimethylphenyl)-4,4′-bipyridinium bis-hexafluorophosphate (2.5 g) formed immediately and was filtered and dried.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com