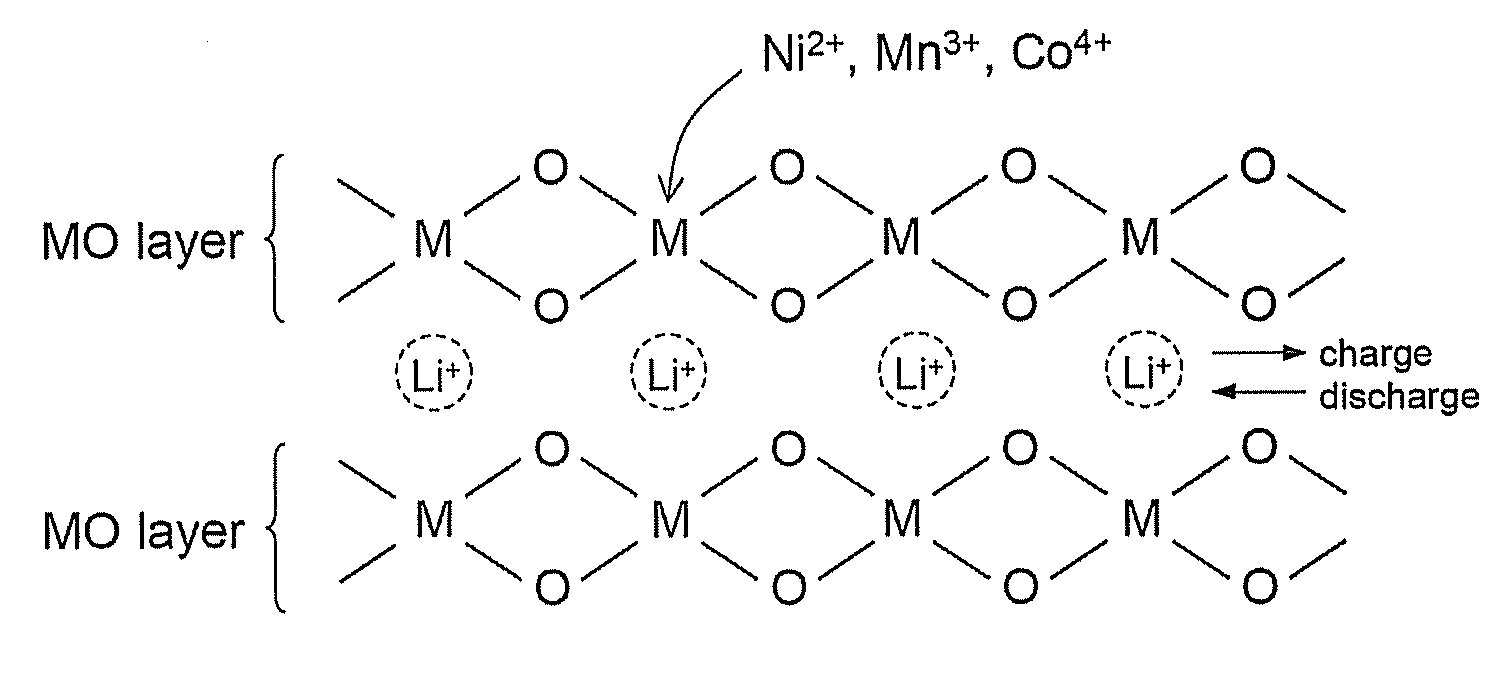
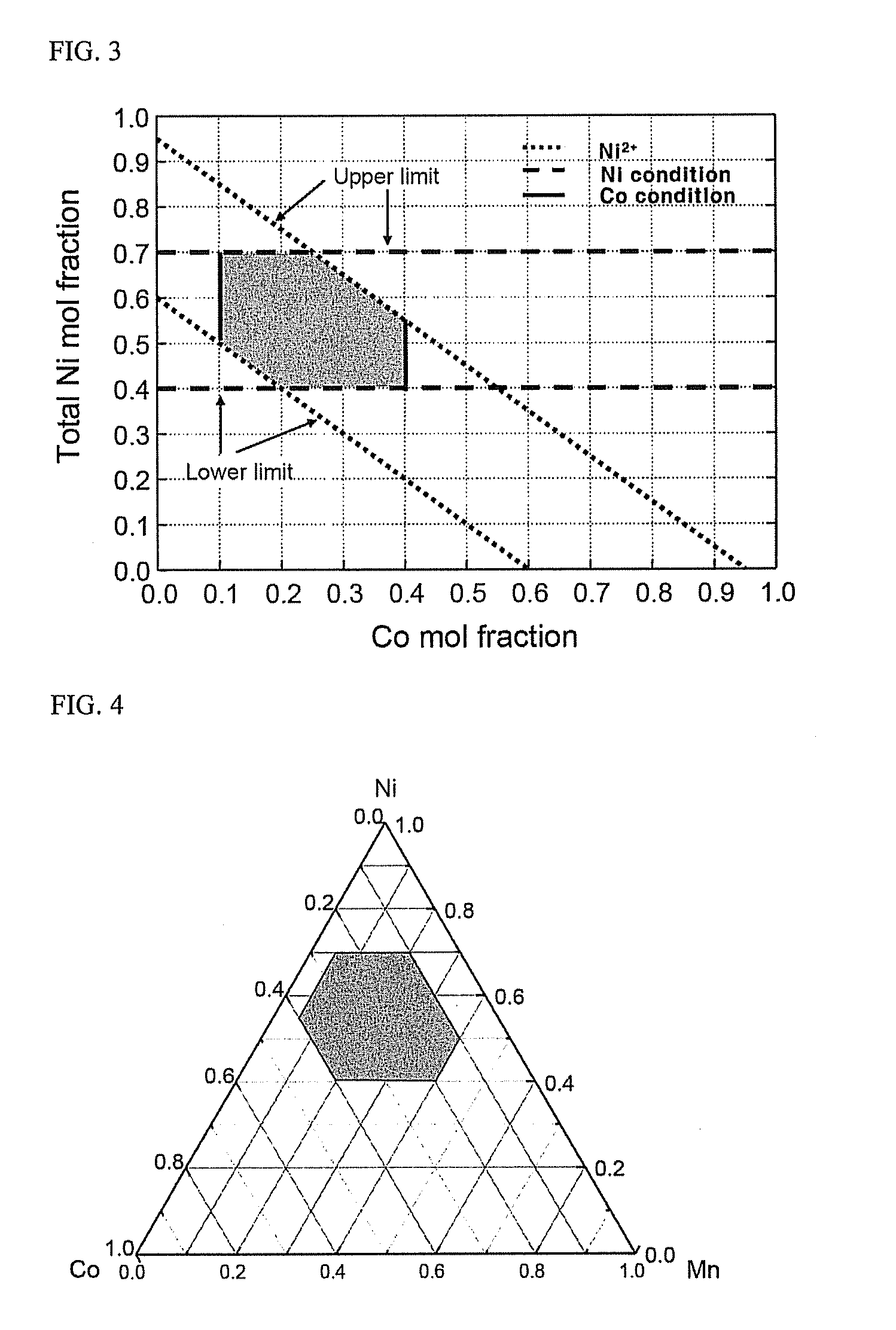
Material for lithium secondary battery of high performance
a secondary battery and lithium mixed technology, applied in nickel compounds, cell components, sustainable manufacturing/processing, etc., can solve the problems of low safety, limited practical and mass application of lithium manganese oxide as a power source, and shortcomings of lithium manganese oxide, etc., to achieve high cycle stability, high capacity, and superior thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0110] A mixed hydroxide of Formula MOOH (M=Ni4 / 15(Mn1 / 2Ni1 / 2)8 / 15Cu0.2) as a mixed transition metal precursor and Li2CO3 were mixed in a stoichiometric ratio (Li:M=1.02:1), and the mixture was sintered in air at various temperatures of 850 (Ex. 1A), 900 (Ex. 1B), 950 (Ex. 1C), and 1,000° C. for 10 hours, to preparing a lithium mixed transition metal oxide. Herein, secondary particles were maintained intact without being collapsed, and the crystal size increased with an increase in the sintering temperature.
[0111] X-ray analysis showed that all samples have a well-layered crystal structure. Further, a unit cell volume did not exhibit a significant change with an increase in the sintering temperature, thus representing that there was no significant oxygen-deficiency and no significant increase of cation mixing, in conjunction with essentially no occurrence of lithium evaporation.
[0112] The crystallographic data for the thus-prepared lithium mixed transition metal oxide are given in...
example 2
[0119] The pH titration was carried out for a sample of the lithium mixed transition metal oxide in accordance with Example 2 prior to exposure to moisture, and samples stored in a wet chamber (90% RH) at 60° C. in air for 17 hours and 3 days, respectively. The results thus obtained are given in FIG. 9. Upon comparing the lithium mixed transition metal oxide of Example 2 (see FIG. 9) with the sample of Comparative Example 3 (see FIG. 8), the sample of Comparative Example 3 (stored for 17 hours) exhibited consumption of about 20 mL of HCl, whereas the sample of Example 2 (stored for 17 hours) exhibited consumption of 10 mL of HCl, thus showing an about two-fold decrease in production of the water-soluble bases. Further, in 3-day-storage samples, the sample of Comparative Example 3 exhibited consumption of about 110 mL of HCl, whereas the sample of Example 2 exhibited consumption of 26 mL of HCl, which corresponds to an about five-fold decrease in production of the water-soluble bases...
example 3
[0121] A mixture of Li2CO3 with mixed hydroxide of Formula MOOH (M=Ni4 / 15(Mn1 / 2Ni1 / 2)8 / 15Co0.2) was introduced into a furnace with an about 20 L chamber and sintered at 920° C. for 10 hours, during which more than 10 m3 of air was pumped into the furnace, thereby preparing about 5 kg of LiNiMO2 in one batch.
[0122] After sintering was complete, a unit cell constant was determined by X-ray analysis, and a unit cell volume was compared with a target value (Sample B of Example 1: 33.921 Å3). ICP analysis showed that a ratio of Li and M is very close to 1.00, and the unit cell volume was within the target range. FIG. 10 shows an SEM image of the thus-prepared cathode active material and FIG. 11 shows results of Rietveld refinement. Referring to these drawings, it was found that the sample exhibits high crystallinity and well-layered structure, a mole fraction of Ni2+ inserted into a reversible lithium layer is 3.97%, and the calculated value and the measured value of the mole fraction o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com