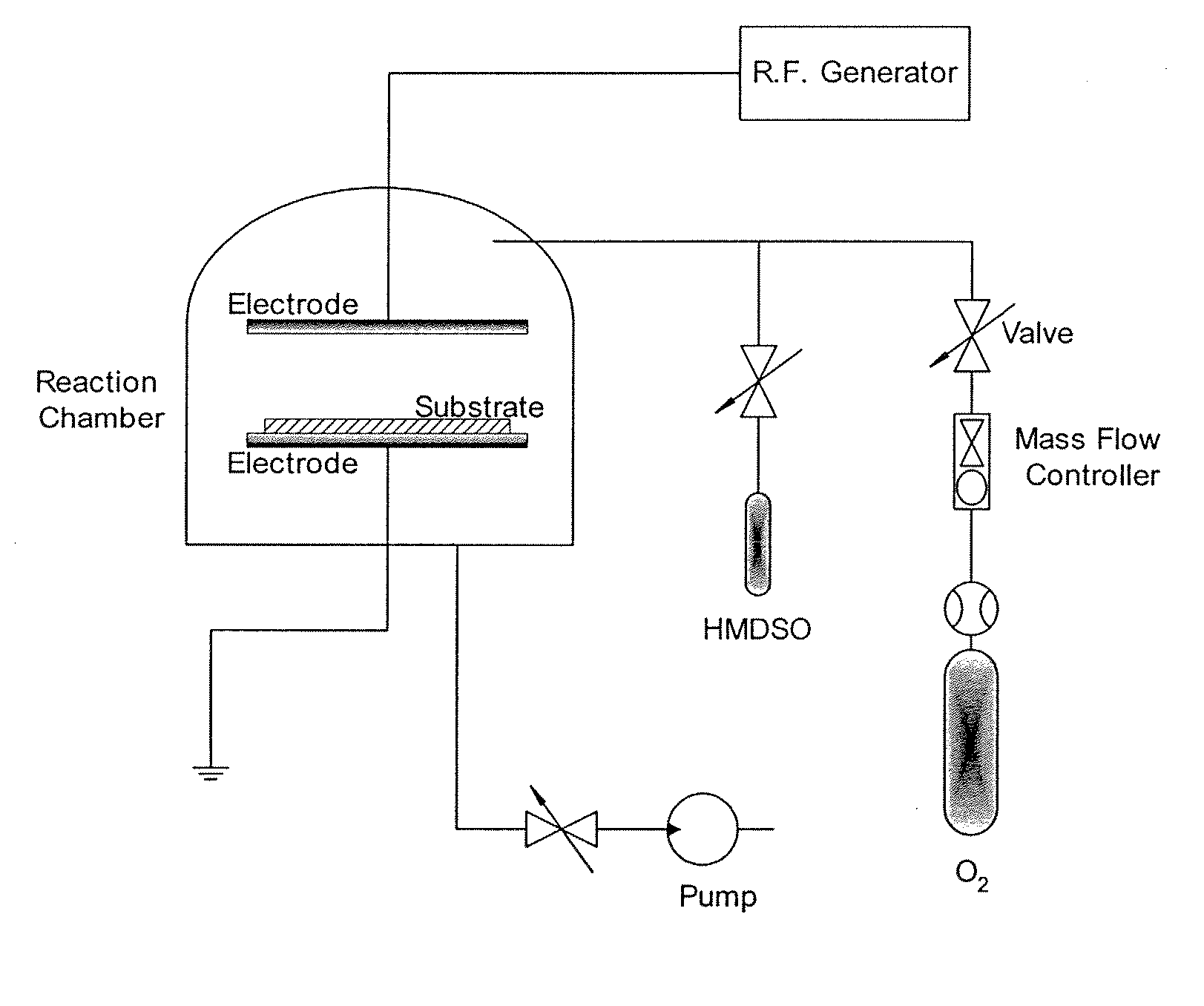
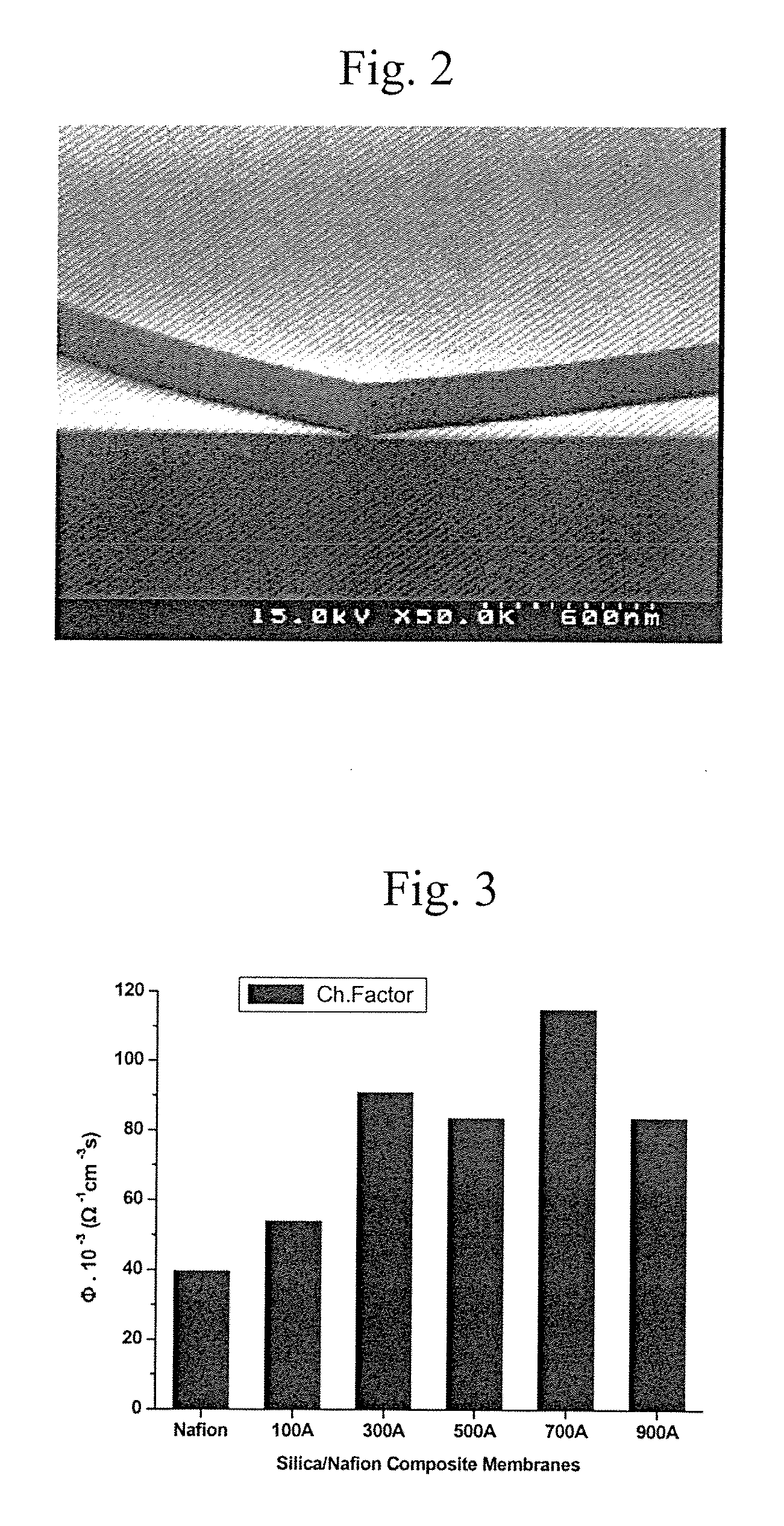
Method to manufacture composite polymer electrolyte membranes coated with inorganic thin films for fuel cells
a fuel cell and inorganic thin film technology, applied in sustainable manufacturing/processing, non-aqueous electrolyte cells, cell components, etc., can solve the problems of degradation of cell performance, excessive consumption of expensive platinum catalysts and gradual performance degradation, and low electrode performance, so as to reduce ionic conductivity, reduce methanol permeability, and reduce the effect of permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
[0059] A composite polymer electrolyte membrane coated with inorganic thin films was manufactured by coating with silica to a thickness of 10 nm on the surface of a Nafion® 115 membrane (Du Pont) via a PECVD method which uses silicon ethoxide (Product of Aldrich) as reactants. For composite polymer electrolyte membrane thus manufactured, the ionic conductivity was 0.091 S / cm and the methanol permeability was 1.68×10−6 cm2 / sec (see Tables 1 and 2 below).
embodiment 2
[0060] A composite polymer electrolyte membrane coated with inorganic thin films was manufactured by coating with silica to a thickness of 30 nm on the surface of a Nafion® 115 membrane (Du Pont) via a PECVD method which uses silicon ethoxide (Product of Aldrich) as reactants. For composite polymer electrolyte membrane for fuel cells thus fabricated, the ionic conductivity was 0.075 S / cm and the methanol permeability was 8.25×10−7 cm2 / sec (see Tables 1 and 2 below).
embodiment 3
[0061] A composite polymer electrolyte membrane coated with inorganic thin films was manufactured by coating with silica to a thickness of 50 nm on the surface of a Nafion® 115 membrane (Du Pont) via a PECVD method which uses silicon methoxide (Product of Aldrich) as reactants. For composite polymer electrolyte membrane thus manufactured, the ionic conductivity was 0.076 S / cm and the methanol permeability was 9.09×10−7 cm2 / sec (see Tables 1 and 2 below).
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| microwave power | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com