Method and apparatus for obtaining enhanced production rate of thermal chemical reactions
a technology of thermal chemical reaction and production rate, which is applied in the direction of gas-gas reaction process, bulk chemical production, physical/chemical process catalyst, etc., can solve the problems of limited production rate, limited heat transfer rate, and longer residence time, and achieve enhanced production rate, enhanced heat transfer rate, and enhanced production rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
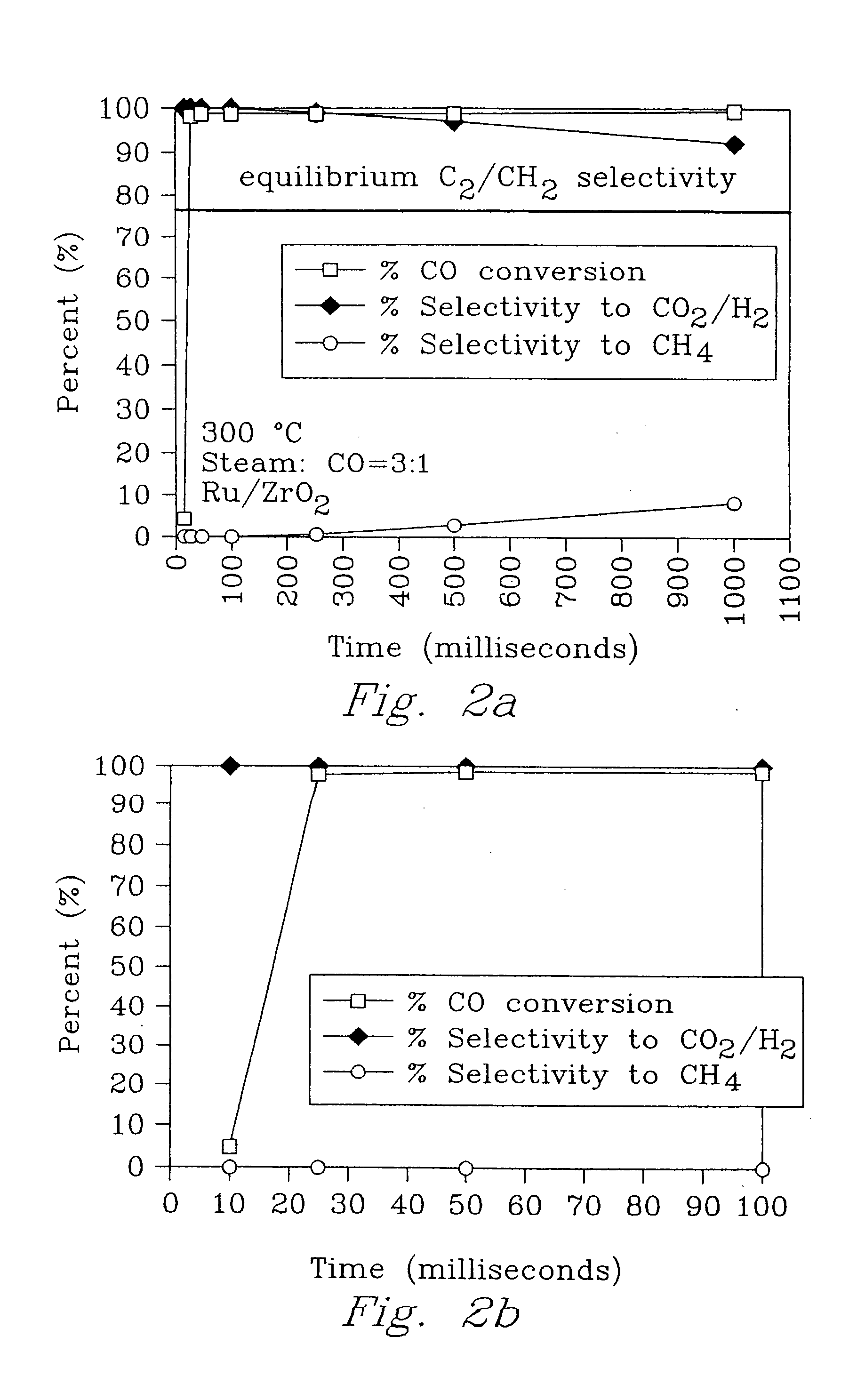
[0065] An experiment was conducted to demonstrate a chemical thermal reactor according to the present invention using the water gas shift reaction.
[0066] A first porous insert was made with a catalyst material of a pre-reduced and stabilized 5-wt % Ru / ZrO2 catalyst (⅛-inch extrudates) obtained from Degussa Corporation. The catalyst material was ground and sieved to 65 to 100 mesh.
[0067] A second porous insert was made with Ni metal foam with 80 pores per inch (ppi) machined to fit in a 7 mm ID quartz tube, ranging from 0.5 to 2.5 cm in length. The metal foam was washed in a sonicator with acetone, chloroform, and water successively over 10-minute intervals. It was also etched in a 1 M HCl solution at 60° C. for 30 min. The etched metal foam was saturated with a zirconium n-propoxide / 1-propanol solution (Aldrich), followed by ambient hydrolysis with water vapor for 72 h, then calcined at 450° C. for 4 h to form the interfacial layer. The ZrO2-coated metal foam was saturated with a ...
example 2
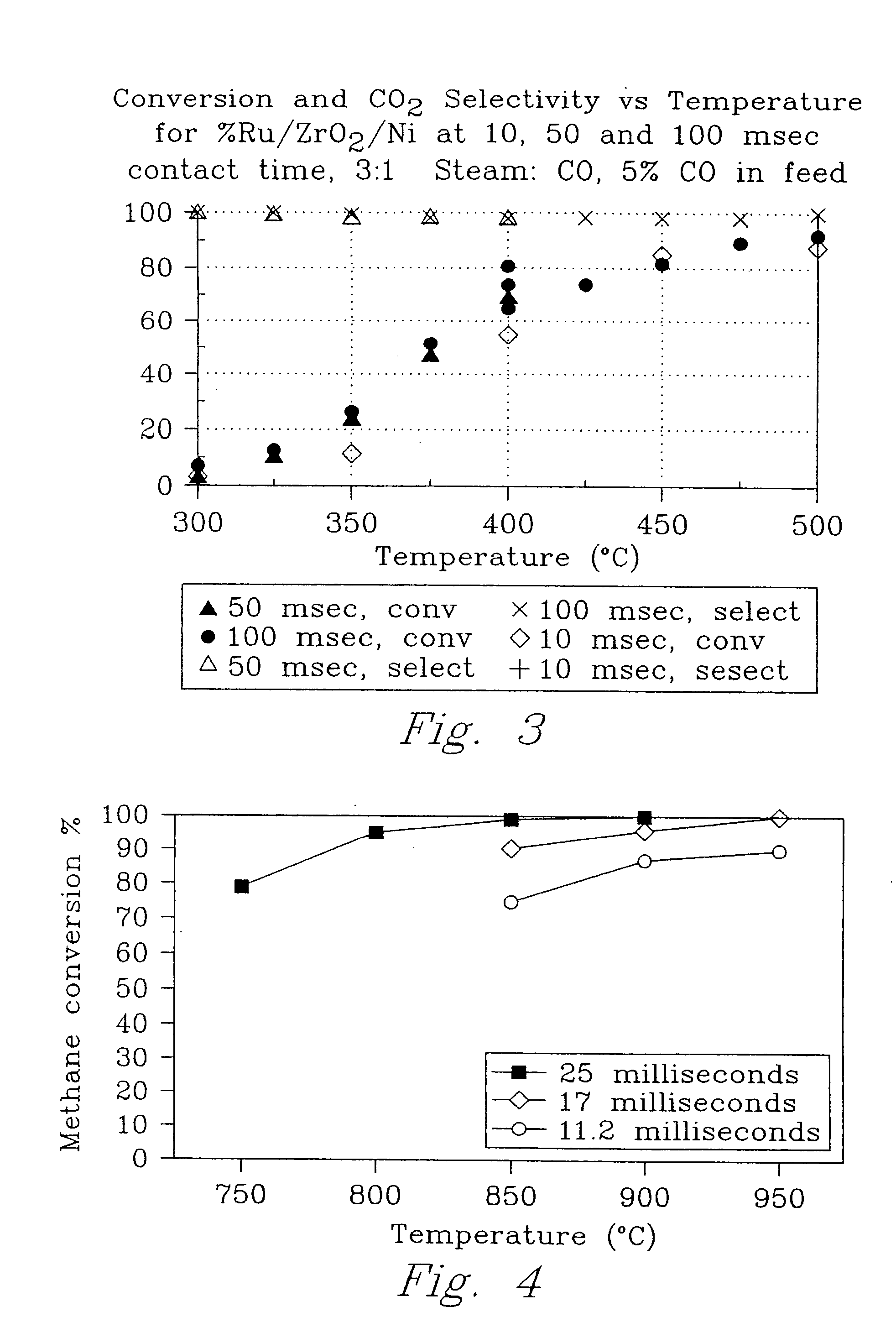
[0077] An experiment was conducted to demonstrate hydrocarbon steam reforming according to the present invention.
[0078] Using the first porous insert (powder) as in Example 1, methane steam reforming was achieved with 100% conversion at 850° C. in 25 milliseconds on a 5% Rh / gamma-Al2O3 catalyst (FIG. 4). Using the second porous insert (coated metal foam) as in Example 1, with a 5% Rh / Al2O3 catalyst / interfacial layers on 80 ppi stainless steel metal foam reduced the operating temperature by 100° C. to achieve the same performance at 750° C.
[0079] No coke formation was observed during any of the millisecond residence time experiments with lower steam to methane ratios (2.5:1).
[0080] Results for other hydrocarbons are shown in Table E2-1 wherein “time” is residence time. The data on butane, gasoline and kerosene were obtained using a powder catalyst while the data on isooctane were obtained using a foam catalyst.
[0081] Table E2-1: Preliminary Hydrocarbon Reforming Data Based on 5% ...
example 3
[0083] A preferred, contemplated, embodiment of the invention is shown in FIG. 6. The distance from the heat source to heat sink is about 1 centimeter or less. This distance is a function of the heat duty, the selection of heat transfer fluid(s), and the effective thermal conductivity of the porous catalyst insert. The porous catalyst insert may have a porosity greater than 95%, which creates an effective thermal conductivity roughly two orders of magnitude below the pure metal or alloy forming the porous support.
[0084] Thin sheets or tubes can be used to obtain high heat duties and short contact times. The thickness of the web between the reaction channel and the heat exchange channel can vary, but is preferably between about 0.01 inches and about 0.25 inches. The preferred thickness for the heat exchange channel preferably ranges from 100 microns to 10 millimeters. The preferred thickness is 250 microns to 3 millimeter. Flow of the heat transfer fluid may be either counter-curren...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| mass transport distance | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com