Production of recombinant polypeptides by bovine species and transgenic methods
a technology of recombinant polypeptides and bovine species, which is applied in the fields of peptides, enzymology, transferrins, etc., can solve the problems of little, if any, success in producing transgenic cows, and achieve the effects of facilitating identification of successful transgenesis, facilitating integration, and resisting digestion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of a Probe Specific for Bovine αS1 Casein Sequences
A. Isolation of Chromosomal DNA
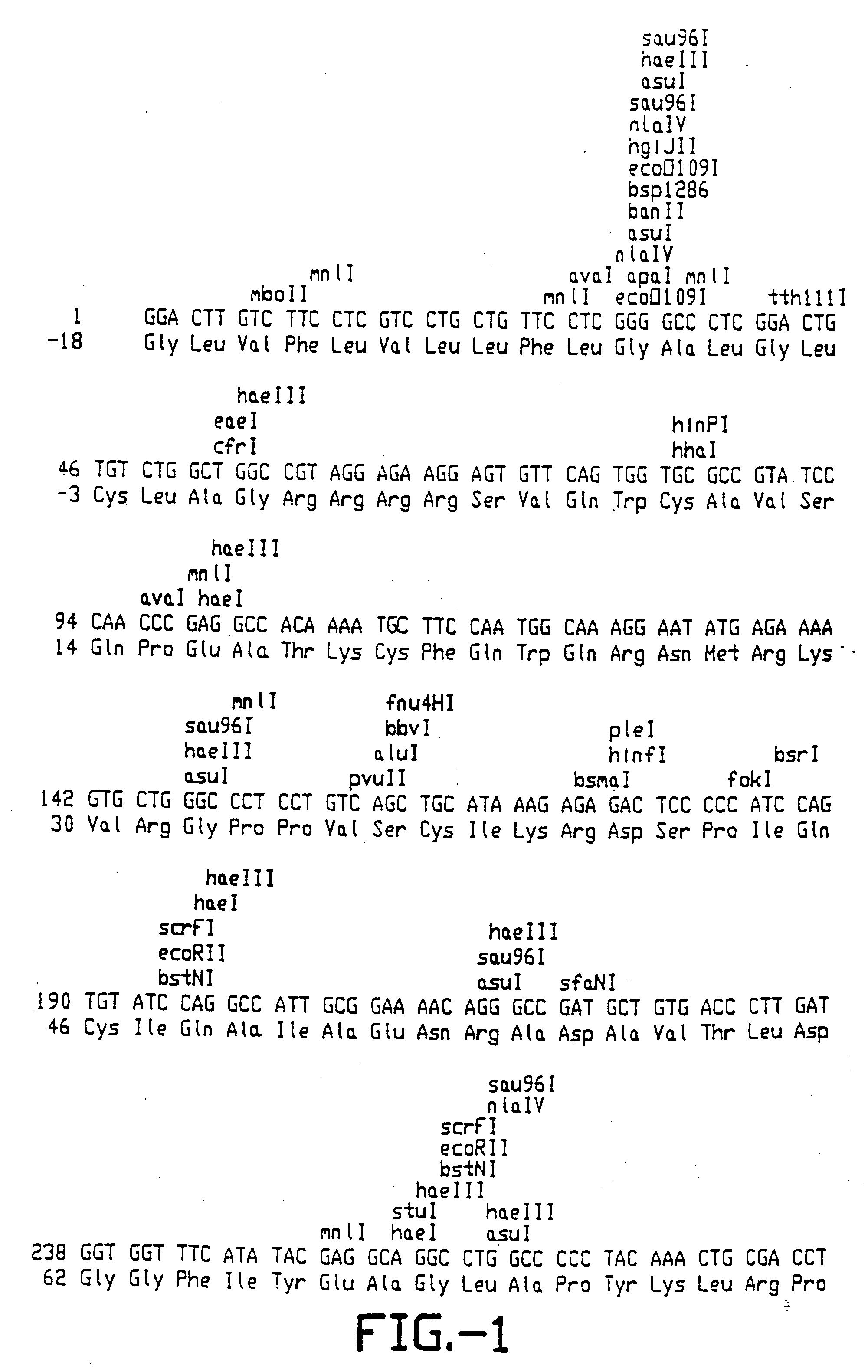
[0118] Placental tissue was obtained from the slaughterhouse. Surrounding connective tissue was removed and pieces of about 30 grams were quickly frozen in liquid N2. Chromosomal DNA was isolated as follows: 30 grams of tissue was homogenized (on ice) with 35 ml of Buffer 1 containing 300 mM Sucrose; 60 mM KCl; 15 mM NaCl; 60 mM Tris.HCl pH 8.2; 0.5 mM spermidine; 0.15 mM spermine; 2 mM EDTA; 0.5 mM EGTA. 65 ml of icecold buffer 1 containing 1% NP40 was added and the mixture was incubated for five minutes on ice. After centrifugation for five minutes at 3000×g the pellet was rinsed with buffer 1 containing 1% NP40. After repeating the centrifugation step the pellet was resuspended in 5 ml of buffer 1. 5 ml 0.5 M EDTA was quickly added. Final volume was now 15 ml. 0.15 ml of a 10% SDS solution was added. After mixing, RNAse A and T1 were added to final concentrations of 0.4 mg / ml and 6 u...
example 2
Cloning of Human Lactoferrin Gene
A. Materials
[0130] Restriction endonucleases, T4 ligase, and T7 polynucleotide kinase were obtained from Boehringer-Mannheim, New England Biolabs, or Bethesda Research Laboratories. Radio-isotopes were purchased from Amersham. A human mammary gland cDNA library in bacteriophage λgt11 was obtained from Clontech, Inc., Palo Alto, Calif.
B. Isolation of the Human Lactoferrin Gene
[0131] The human mammary gland library was screened by standard plaque hybridization technique (Maniatis, et al. (1982) Molecular Cloning: A Laboratory Manual) with three synthetic oligomers. Two of the oligomers were 30-mers corresponding to the cDNA sequence of Rado et al., supra, at amino acid positions 436-445 and 682-691. The third was a 21-mer “best guess” probe based on human codon bias and coding for amino acid sequence of HLF between amino acid residues 18 and 24. Respectively, they were:
(Seq. ID No.: 9)(1)5′-CTTGCTGTGGCGGTGGTTAGGAGATCAGAC-3′(Seq. ID No.: 10)(2)5...
example 3
Construction of bovine αS1-casein CAT vectors
[0134] In order to determine whether the αS1 casein fragments obtained in Example 1 had promoter and other properties needed to express a heterologous gene, expression plasmids were constructed containing variable amounts of 5-′ and 3′-flanking regions from the αS1-casein gene. The chloramphenicol Acetyl transferase gene (CAT) was used as a heterologous gene in these vector constructs. The CAT gene is useful to detect the expression level for a heterologous gene construct since it is not normally present in mammalian cells and confers a readily detectable enzymatic activity (see Gorman, C. N., et al. (1983), Mol. Cell. Biol. 2, 1044-1051) which can be quantified in the cells or animals containing an expressible gene.
A. DNA Sequences
[0135] 681 bp of a αS1-casein promoter plus the first non-coding exon plus approximately 150 bp of the first intervening sequence (IVS) were isolated from a 5′-flanking genomic clone from Example 1 by PCR a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com