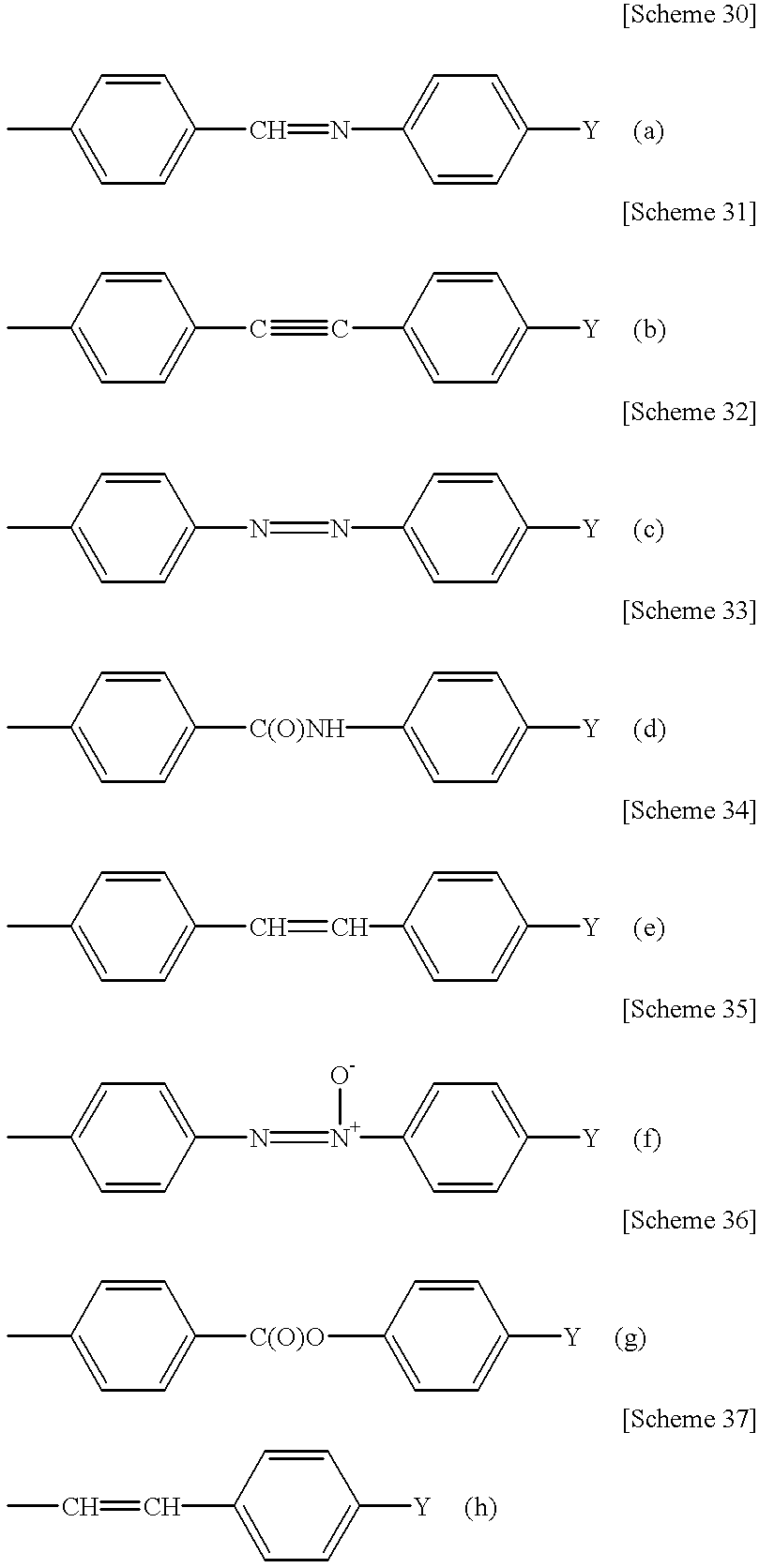
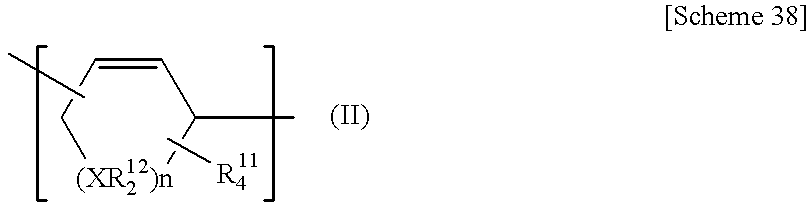Poly (cyclic conjugated diene) and process for producing the same
a conjugated diene and polymer technology, applied in the field of poly (cyclic conjugated diene), can solve the problems of inability to obtain sufficient polymer yield, inability to polymerize a cyclic conjugated diene monomer having a polar functional group, and inability to synthesize copolymers. achieve high yield, high heat resistance and oxidation resistance, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0335] The catalyst A (109 mg, 0.1 mmol) previously synthesized was dissolved into 15 ml of toluene at room temperature under an argon atmosphere in a glove box, to adjust a catalyst solution. In the meantime, 1,3-cyclohexadiene (8 g, 0.1 mol) which is a cyclic conjugated diene monomer was dissolved into 15 ml of toluene at room temperature under an argon atmosphere in a glove box. While this solution was kept to room temperature and was stirred, the total amount thereof was added to the total amount of the above-mentioned catalyst solution. Polymerization advanced promptly so that a powdery polymer was precipitated from the toluene solution. The polymerizing reaction finished within 30 minutes. A slurry containing the produced polymer was taken out from the glove box, and then was added to a great deal of methanol acidified with hydrochloric acid to precipitate and isolate the polymer. Methanol was then removed by filtration. The resultant was vacuum-dried at room temperature for 2...
example 2
[0337] The catalyst B (18 mg, 0.1 mmol) previously synthesized was dissolved into 15 ml of toluene at room temperature under an argon atmosphere in a glove box, and then 1.8 ml of a toluene solution (aluminum: 10 atomic %) of methyl aluminoxane which is a co-catalyst to adjust a catalyst solution. Thereafter, the same operation as in Example 1 was conducted to polymerize 1,3-cyclohexadiene. The yield of the resultant 1,3-cyclohexadiene was 75%.
[0338] The polymer was a white powder, and insoluble in organic solvents. According to an element analysis, carbon was 90% and hydrogen was 10%. This was satisfactorily consistent with the calculation values based on the monomers. The temperature at which thermal weight loss started was 330.degree. C.
example 3
[0339] The catalyst B (0.5 g) previously synthesized was dissolved into 100 ml of toluene at room temperature under an argon atmosphere in a glove box, and then 50 ml of a toluene solution (aluminum: 10 atomic %) of methyl aluminoxane which is a co-catalyst was added thereto so as to adjust a catalyst solution.
[0340] 50 g of 1,3-cyclohexadiene was dissolved into 200 ml of toluene at room temperature. While this solution was kept to room temperature, the total amount thereof was added to the total amount of the above-mentioned catalyst solution.
[0341] At the same time of the addition, a polymerizing reaction advanced at room temperature at a stroke to obtain polycyclohexadiene. The time for the polymerization was 15 seconds. The yield was 82%.
[0342] The polymer was a white powder, and insoluble in organic solvents. According to an element analysis, carbon was 90% and hydrogen was 10%. This was satisfactorily consistent with the calculation values based on the monomers. The temperatur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com