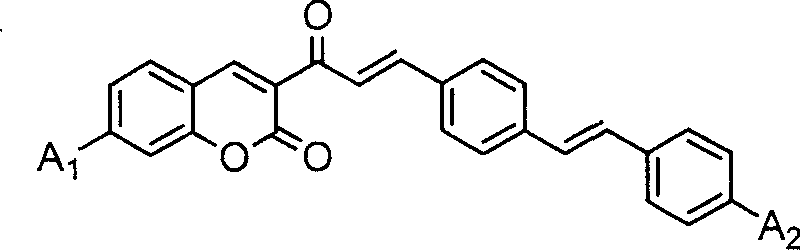
Coumarin dye connected by diphenyl ethylene and its synthesis method and use
A technology of coumarin dyes and coumarins, which is applied in the direction of coumarin dyes, etc., can solve the problem of low two-photon absorption cross section, and achieve the effects of simple synthesis and separation methods, high yield, and easy-to-obtain raw material sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] A 1 、A 2 Synthesis of coumarin dyes (K1) with diethylamino groups
[0060] (1) Add 10 mmoles of 3-acetyl-7-diethylaminocoumarin, 20 mmoles of terephthalaldehyde and 50 ml of glacial acetic acid into a 100 ml three-necked flask, stir well, add 0.5 ml of hexahydropyridine, and heat Reflux at 120°C for 12 hours. After the reactant was cooled, it was filtered with suction, and the filter cake was washed with 10 ml of ethanol. The crude product was recrystallized twice with ethanol / acetonitrile (m / m=1:1) mixed solvent to obtain 3-[3-(4-formaldehyde)phenyl]acryloyl-7-diethylaminocoumarin intermediate body with a yield of 64.8%.
[0061] (2) 50 mmoles of potassium iodide, 50 mmoles of triphenylphosphine, 50 mmoles of diethylaniline, 3.75 g of 40% aqueous formaldehyde solution, 40 ml of chloroform, 20 ml of water, and 10 g of glacial acetic acid were mixed in a 250 ml round-bottomed flask, and kept at room temperature The mixture was stirred under low temperature for 3 wee...
Embodiment 2
[0064] Synthesis of Coumarin Dye (K2) Both A1 and A2 Are Methoxy
[0065] (1) Add 20mmol of 3-acetyl-7-methoxycoumarin, 50mmol of terephthalaldehyde and 90ml of glacial acetic acid into a 100ml three-necked flask, stir well, add 2ml of hexahydropyridine, and heat to 120 °C reflux for 25 hours. After the reactant was cooled, it was suction filtered, and the filter cake was washed with 10 ml of ethanol. The crude product was recrystallized twice with 30 ml of ethanol / acetonitrile (m / m=1:1) mixed solvent to obtain 3-[3-(4-formaldehyde)phenyl]acryloyl-7-methoxycoumarin Intermediate, yield 85.2%
[0066] (2) 10 millimoles of p-methoxybenzyl chloride and 10 millimoles of triphenylphosphine were added to a 100ml round-bottomed flask, and 30 milliliters of cyclohexane was added, heated to reflux for 2 hours, cooled to room temperature after the reaction, and pumped After filtering, the filter cake was washed with 15ml of ether to obtain the p-methoxybenzyl chlorophosphonate salt, w...
Embodiment 3
[0069] A 1 For dimethylamino, A 2 Synthesis of Diethylamino Coumarin Dye (K3)
[0070] (1) In a 100ml there-necked flask, add 20 mmoles of 3-acetyl-7-dimethylaminocoumarin, 50 mmoles of terephthalaldehyde and 50 ml of acetonitrile / ethanol mixed solvent with a volume ratio of 1: 1, stir After uniformity, add 1ml of acetic acid and 1ml of hexahydropyridine, and heat to reflux for 30 hours. After the reactant was cooled, it was filtered with suction, and the filter cake was washed with 8 ml of acetonitrile / ethanol mixed solvent. The crude product was recrystallized twice with ethanol / acetonitrile (m / m=1:1) mixed solvent to obtain 3-[3-(4-formaldehyde)phenyl]acryloyl-7-dimethylaminocoumarin intermediate body, yield 86.3%
[0071] (2) 0.1 mole of potassium bromide, 0.1 mole of triphenylphosphine, 0.1 mole of diethylaniline, 37.5 g of 40% formaldehyde solution, 150 ml of chloroform, 100 ml of water, and 50 g of glacial acetic acid were mixed in a 500 ml round bottom flask, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com