Phosphoryl choline chitosan derivative synthesis method
A technology for chitosan derivatives and phosphorylcholine, which is applied in the field of synthesis of phosphorylcholine chitosan derivatives, can solve the problem of affecting the specific interaction of scaffolds with biologically active substances, and is not conducive to the specific recognition of cells for tissue growth and application. effects, etc., to achieve the effects of improving biofunctionality and biocompatibility, inhibiting non-specific adsorption, and improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
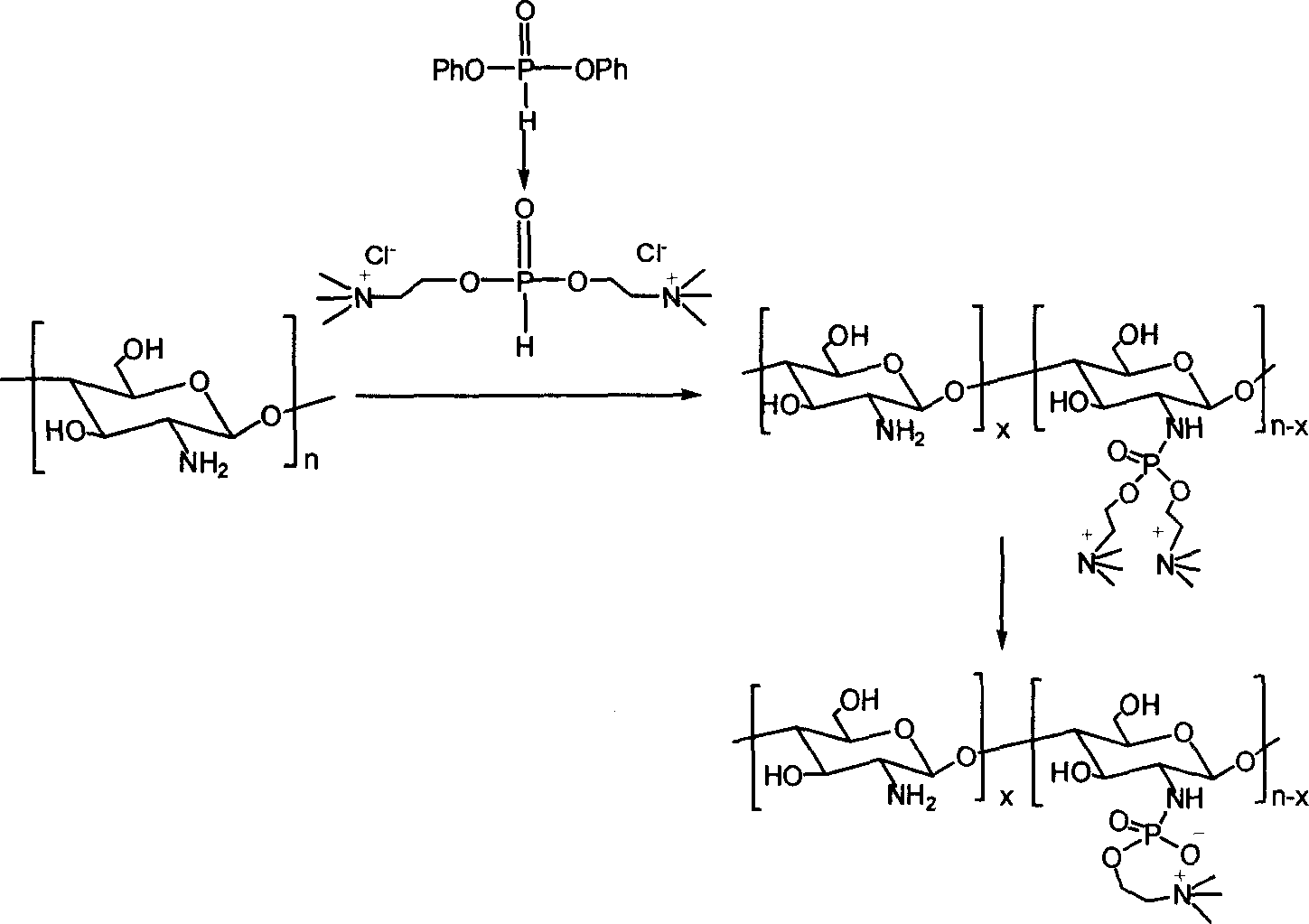
[0020] (1) Preparation of double substituted choline phosphonate
[0021] Dissolve 0.01mol of choline chloride in 30-50ml of dimethyl sulfoxide, heat and dry in a vacuum to remove water completely, then add 0.005mol of p-phenoxyphosphonate and 3-5ml of anhydrous pyridine, at room temperature The reaction was stirred for 2 hours to obtain a disubstituted choline phosphonate solution.
[0022] (2) Preparation of phosphorylcholine chitosan derivatives
[0023] The disubstituted choline phosphonate solution prepared in step (1) was spin-dried to solvent, and dissolved in anhydrous isopropanol to obtain reaction solution A.
[0024] Dry 0.0005mol of chitosan powder with a deacetylation degree of 50% and a molecular weight of 228,000 in a vacuum oven at 50°C for 24 hours, remove the adsorbed water on the surface, and add 3ml of triethylamine and 2ml of carbon tetrachloride for drying treatment. , 5ml of isopropanol mixed solvent as a dispersant to obtain reaction solution B. The ...
Embodiment 2
[0026] (1) Preparation of double substituted choline phosphonate
[0027] Dissolve 0.02 mol of choline chloride in 60-120 ml of dimethyl sulfoxide, heat and dry in vacuum, completely remove water, then add 0.01 mol of p-phenoxyphosphonate and 6-10 ml of anhydrous pyridine, at room temperature The reaction was stirred for 2 hours to obtain the disubstituted choline phosphonate.
[0028] (2) Preparation of phosphorylcholine chitosan derivatives
[0029] The disubstituted choline phosphonate solution prepared in step (1) was spin-dried to solvent, and dissolved in anhydrous 2-butanol to obtain reaction solution A.
[0030] Dry 0.005mol of chitosan powder with a degree of deacetylation of 80% and a molecular weight of 46,000 in a vacuum oven at 50°C for 24 hours to remove the adsorbed water on the surface, and add 6ml of triethylamine and 3ml of carbon tetrachloride for drying treatment. , 10ml of 2-butanol mixed solvent as a dispersant to obtain reaction solution B. At 40° C.,...
Embodiment 3
[0032] (1) Preparation of double substituted choline phosphonate
[0033] Dissolve 0.01mol of choline chloride in 30-50ml of dimethyl sulfoxide, heat and dry in a vacuum to remove water completely, then add 0.005mol of p-phenoxyphosphonate and 3-5ml of anhydrous pyridine, at room temperature The reaction was stirred for 2 hours to obtain a disubstituted choline phosphonate solution.
[0034] (2) Preparation of phosphorylcholine chitosan derivatives
[0035] The disubstituted choline phosphonate solution prepared in step (1) was spin-dried to solvent, and dissolved in anhydrous isopropanol to obtain reaction solution A.
[0036] Dry 0.005 mol of chitosan powder with a deacetylation degree of 100% and a molecular weight of 5000 in a vacuum oven at 50°C for 24 hours to remove the adsorbed water on the surface, and add 3 ml of triethylamine and 1.5 ml of tetrachloride Carbon and 10ml of isopropanol mixed solvent were used as dispersant to obtain reaction liquid B. In an ice-wat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com