Method for preparing epirubicin slow-release prepn
A technology of epirubicin and sustained-release preparation, which is applied in the field of drug-loaded polymer microspheres, can solve the problems of unstable epirubicin and can only be stored, and achieves improved drug release characteristics, high encapsulation efficiency, and ease of use. repeated effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
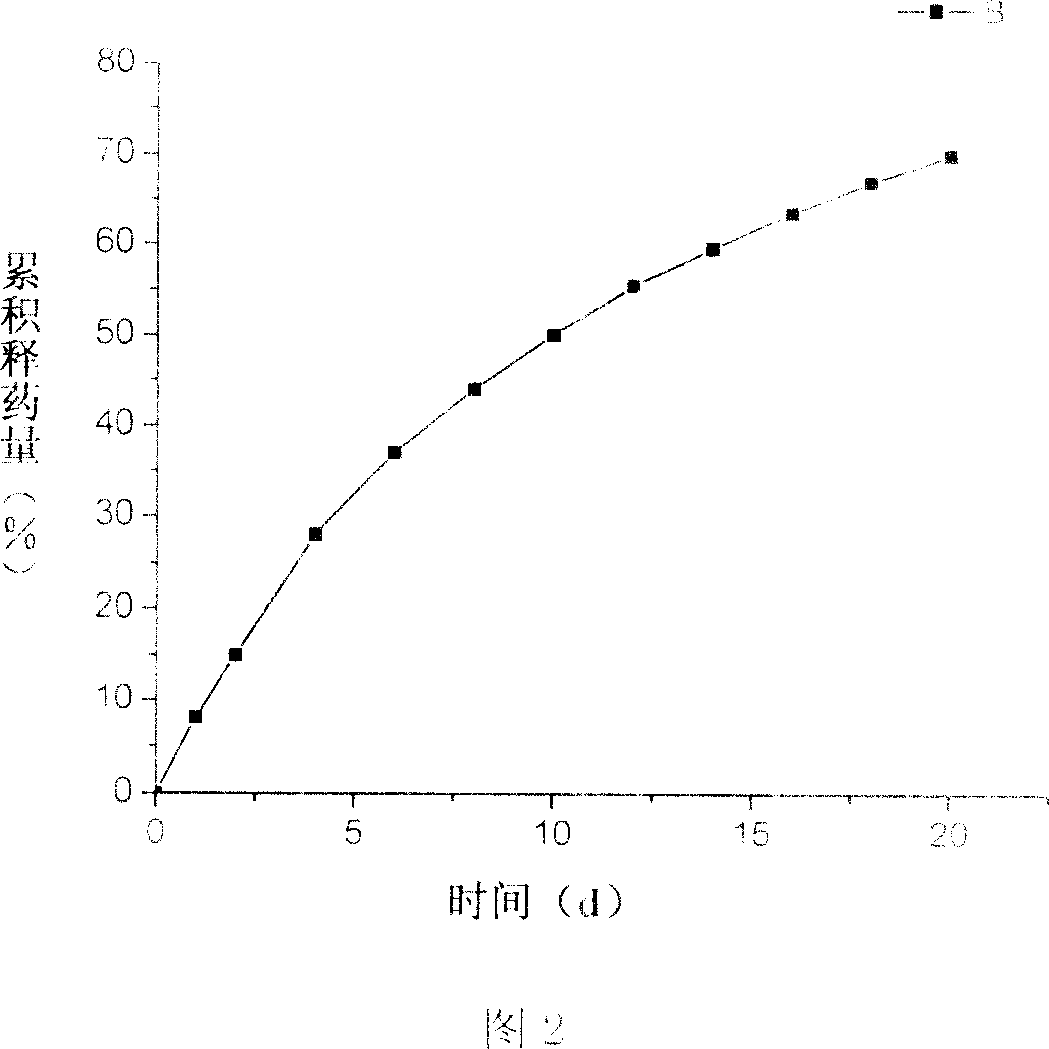
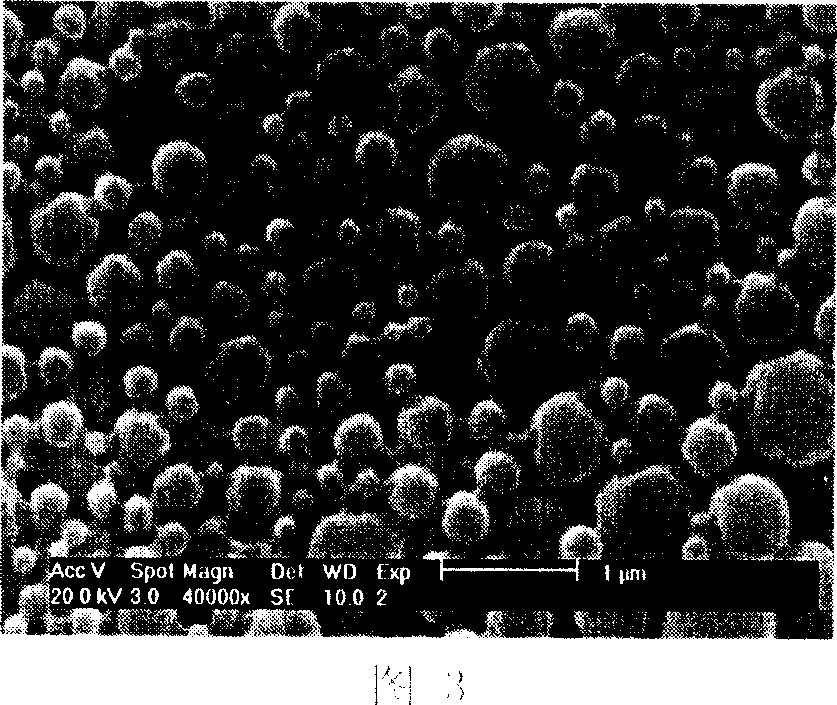
[0024] (1) Dissolve 5 mg of epirubicin hydrochloride in 30 ml of N-N dimethylformamide (DMF) to obtain solution A; (2) take 10 μl of triethylamine and add it to solution A to obtain solution B; (3) weigh 50 mg of polylactic acid (relative molecular weight is 5000) was added to solution B to obtain solution C; (4) 1.6 ml of water was added to solution C to obtain a turbid solution; (5) the turbid solution was transferred to a dialysis bag, and the dialysis Place the bag in distilled water for dialysis, stir at the same time, and change the water every 2 hours; (6) After 8 hours, centrifuge the product obtained in step 5, sonicate, and wash 3 times with water to remove unembedded epirubicin, and take the sample obtained by freeze-drying the precipitate . The encapsulation rate of the obtained sample was measured to be 59.6%, and the drug loading rate was 6.8%. The surface of the microspheres was observed through a scanning electron microscope to be smooth, and the particle size ...
Embodiment 2
[0026] (1) Dissolve 12 mg of epirubicin hydrochloride in 18 ml of N-N dimethylformamide (DMF) to obtain solution A; (2) take 12 μl of triethylamine and add it to solution A to obtain solution B; (3) weigh Add 60mg of polylactic acid (relative molecular weight: 5000) to solution B to obtain solution C; (4) add 2ml of pure water to solution C to obtain a cloudy solution; (5) transfer the cloudy solution to a dialysis bag (8000-14000 ), and place the dialysis bag in distilled water for dialysis, while stirring, and change the water every 2h; (6) after 24h, the product obtained in step 5 is centrifuged, ultrasonicated, and washed 3 times with water to remove unembedded epirubicin, and take The resulting sample was precipitated and freeze-dried. The encapsulation rate of the obtained sample was measured to be 53.6%, and the drug loading rate was 7.4%. The scanning electron microscope observed that the surface of the microspheres was smooth, and the particle size distribution was be...
Embodiment 3
[0028] (1) 4 mg of epirubicin hydrochloride was dissolved in 3 ml of N-N dimethylformamide (DMF) to obtain solution A; (2) 2 μl of triethylamine was added to solution A to obtain solution B; (3) weighed Add 40mg of polylactic acid (relative molecular weight is 10,000) into solution B to obtain solution C; (4) add 0.75ml of pure water to solution C to obtain a cloudy solution; (5) transfer the cloudy solution to a dialysis bag (8000 ~14000), put the dialysis bag in distilled water for dialysis, stir at the same time, change the water every 2h; (5) after 4h, centrifuge the product obtained in step 5, ultrasonic, and wash 3 times with water to remove unembedded epirubicin , take the sample obtained by freeze-drying the precipitate. The encapsulation rate of the obtained sample was 30.9%, and the drug loading rate was 4.9%. The scanning electron microscope observed that the surface of the microspheres was smooth, and the particle size distribution was between 500nm and 1200nm, as ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com