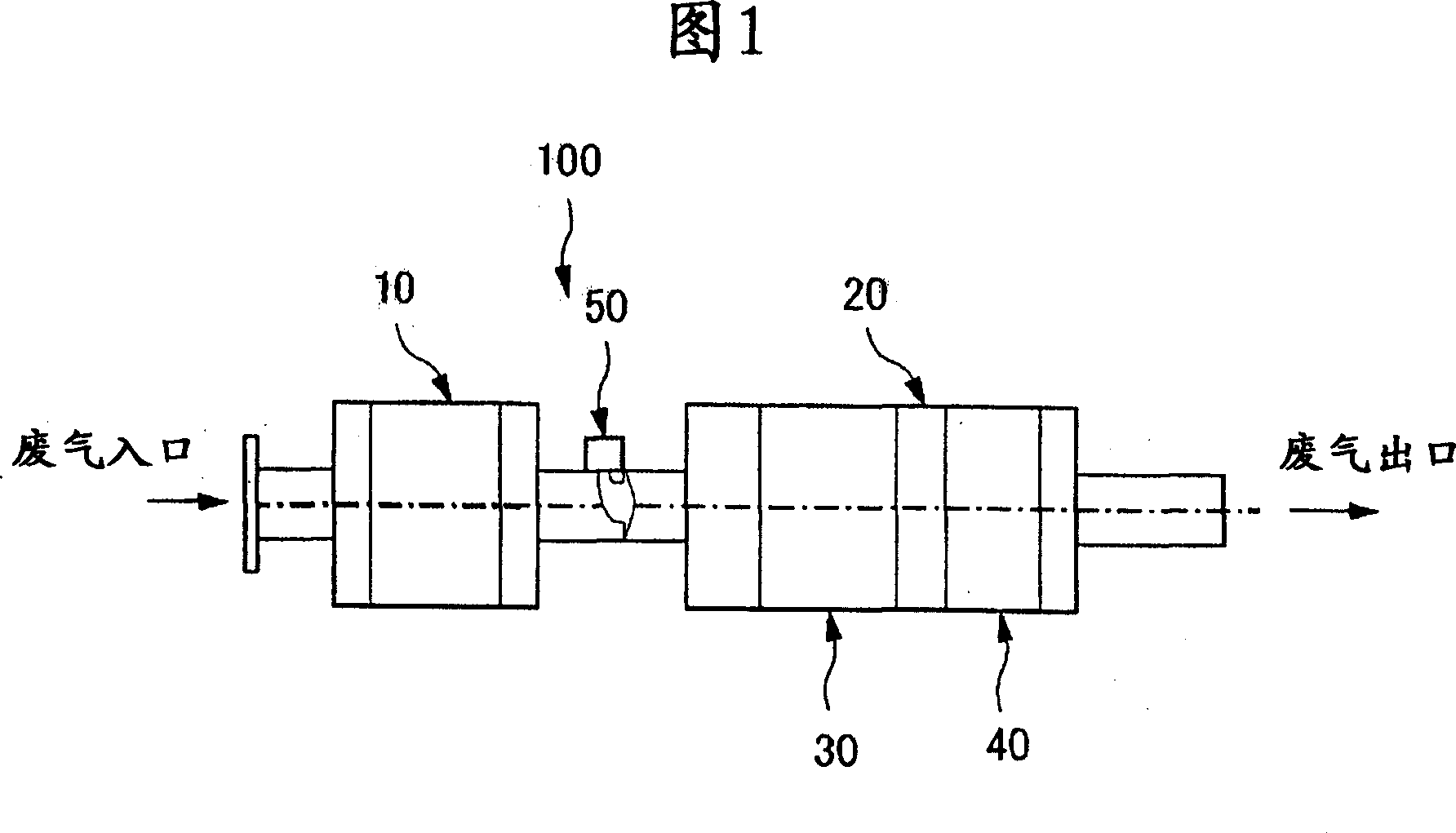
Catalyst system for removing nitrogen oxide and method for removing nitrogen oxide
一种催化剂、氧化氮的技术,应用在化学仪器和方法、催化剂活化/制备、非均相催化剂化学元素等方向,能够解决氨漏出、环境污染等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] 4 Preparation of -Zr-based catalyst>
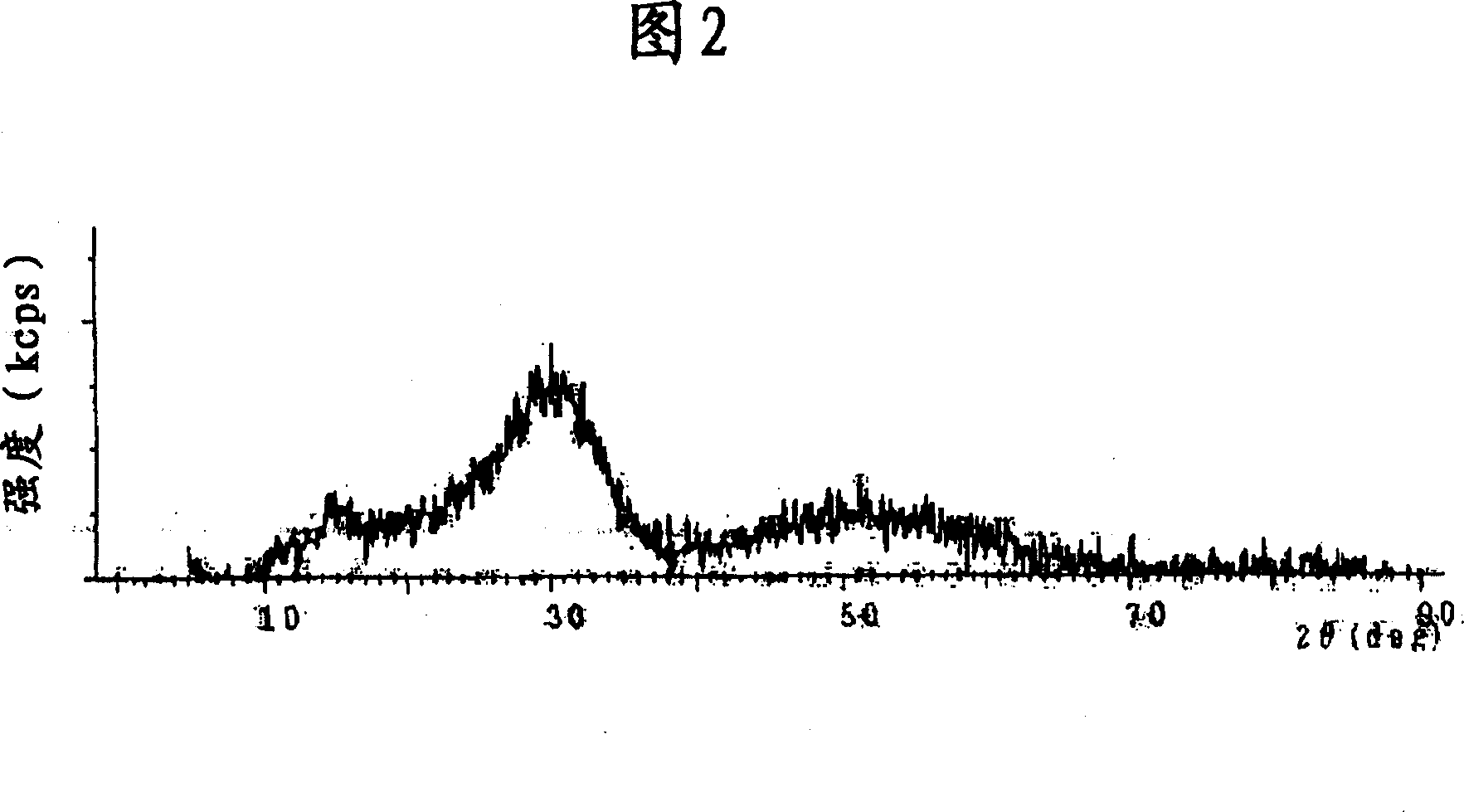
[0087] An aqueous solution of a mixture of 100 g of Zr salt (zirconium sulfate), 50 g of Ti salt (titanium chloride) and 50 g of Ce salt (cerium nitrate) dissolved in 1 L of water was prepared, followed by addition of alkali solution (ammonia) for neutralization and filtration. The solution is burned at 400° C. or higher, and pulverized to obtain a powder. Thereafter, the powder was determined to be Ce-Ti-SO by X-ray diffraction 4 - Zr-based catalysts (see Figure 2).
Embodiment 2
[0089]
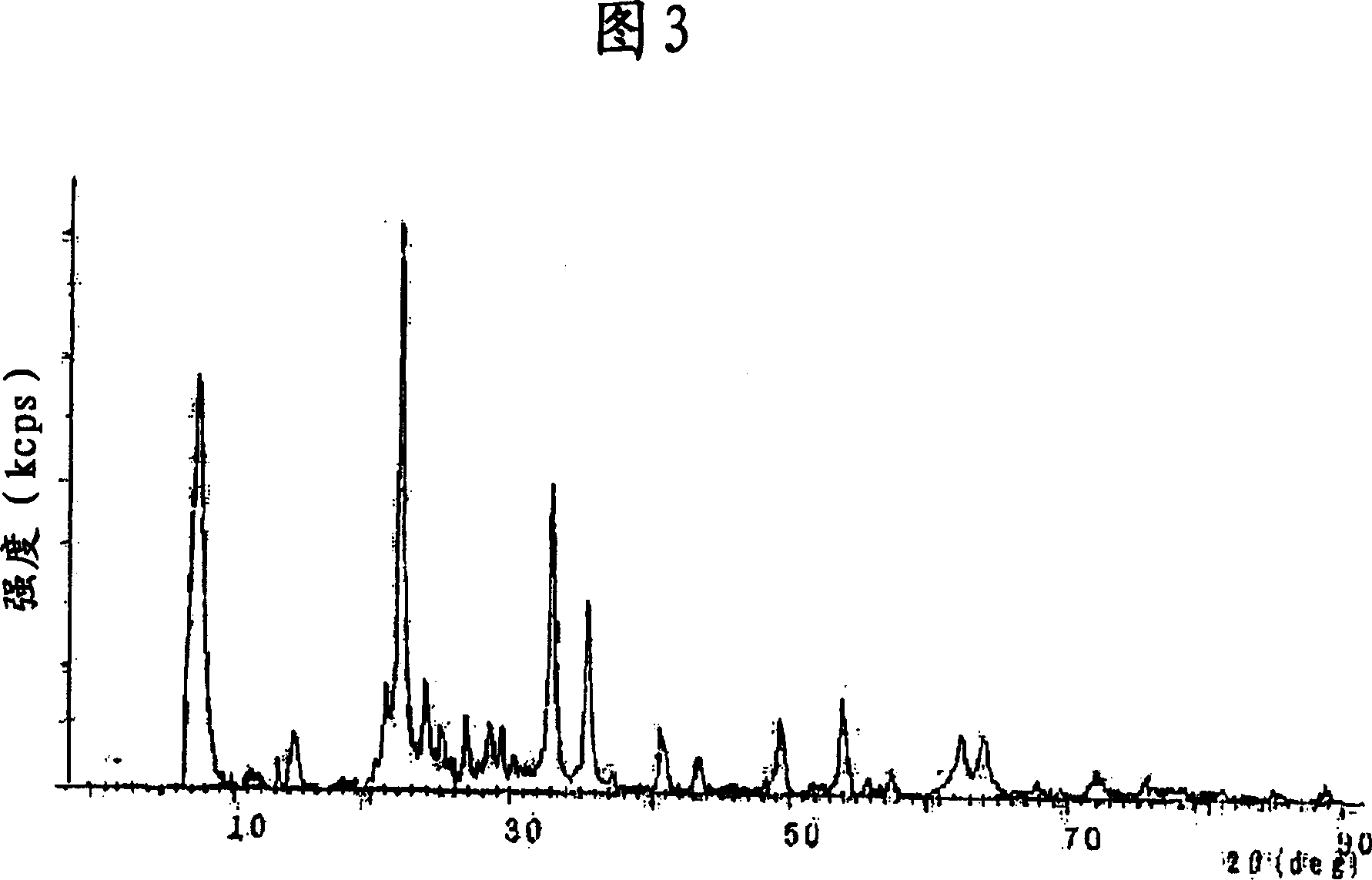
[0090] While stirring, an aqueous solution of ferric nitrate (prepared by dissolving 1,000 g of ferric nitrate in 500 L of water) was gradually dropped onto 1,000 g of porous oxide composed of silica-alumina (molar composition ratio = 40 / 1) superior. The obtained powdery substance was dried at 120° C. and fired at 450° C. for 2 hours to obtain a powder. Thereafter, it was confirmed that the powder was an Fe-Si-Al oxide-based catalyst (see FIG. 3 ).
Embodiment 3
[0092]
[0093] An aqueous solution of a mixture of 100 g of Zr salt (zirconium sulfate) and 50 g of Ce salt (cerium nitrate) dissolved in 1 L of water was prepared, then neutralized by adding an alkali solution (ammonia water), and filtered. 15 g of ammonium tungstate was immersed in the solution, then burned at 400° C. or higher, and pulverized to obtain a powder. Thereafter, it was confirmed that the powder was a Ce-W-Zr oxide-based catalyst (see FIG. 4 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com