Eremophilone lactones acid natural product and application thereof
A technology of lactone acid and lactone, which is applied in the field of natural medicinal chemistry and pharmacology, can solve the problems that the research on biological activity has not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
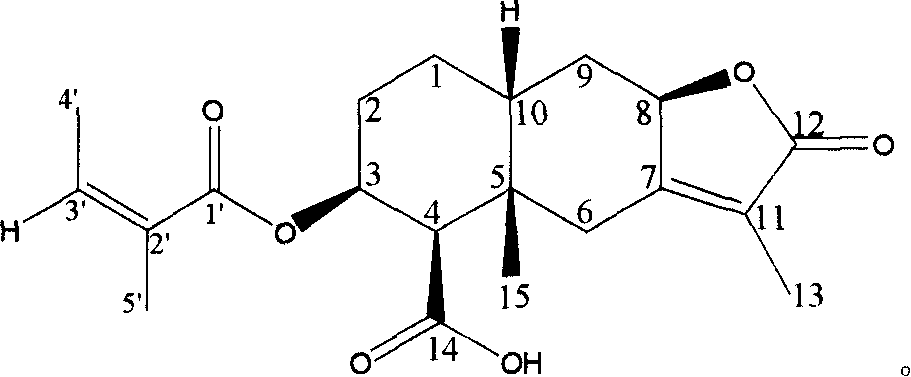
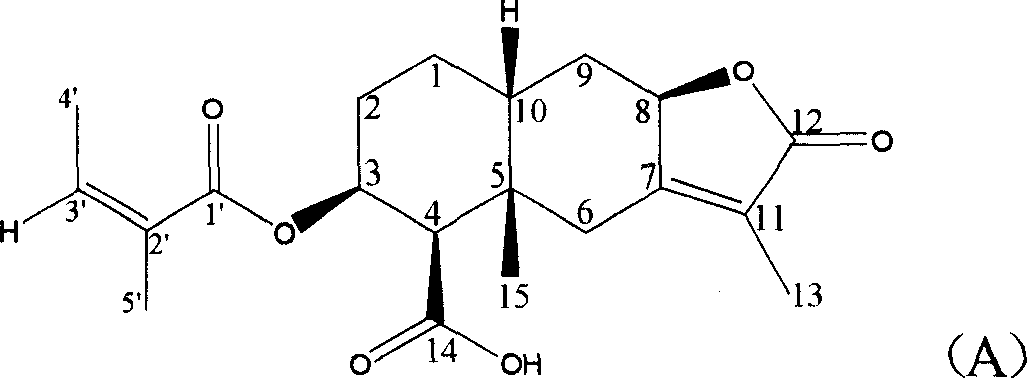
[0020] Embodiment 1: the preparation of compound (A)
[0021] Take 5.0 kg of dry underground part of Ligularia spp., crush it into powder, add 50 liters of 95% ethanol to soak it. Soak 3 times at room temperature, 7 days each time. The ethanol extracts were combined, and the solvent was recovered under reduced pressure to dryness to obtain 462 grams of extract, dispersed with 3 liters of water, and then extracted with 60-90° petroleum ether, ethyl acetate, and n-butanol in sequence. The ethyl acetate extract was recovered under reduced pressure to obtain 89.0 g of extract. The extract was chromatographed on 800 g of 100-200 mesh silica gel column, and chloroform-methanol (50:0-0:1) gradient eluted. TLC detection The same fractions were combined, and the fractions containing compound (A) were concentrated in the 20:1 and 10:1 eluents. The solvent was evaporated to dryness under reduced pressure to obtain 6.9 grams of extract, which was then purified by column chromatography ...
Embodiment 2
[0025] Embodiment 2: the cytotoxic activity of compound (A) to KB cell
[0026] KB (oral epithelial carcinoma) cells were cultured with RPMI 1640 medium containing 10% fetal bovine serum, 100 U / mL penicillin and 100 U / mL streptomycin. cells in 5×10 per well 3 concentration into a 96-well plate at 37 °C with 5% CO 2 Incubate for 24 hours in a humidified incubator.
[0027]Cell viability was determined by the modified MTT method. After the cells were incubated for 24 hours, the newly prepared dimethyl sulfoxide solution of compound (A) was added to each well in a concentration gradient, so that the final concentrations of compound (A) in the wells were 100 μg / mL, 33.3 μg / mL, respectively. mL, 11.1 μg / mL and 3.7 μg / mL. After 72 hours, add 10 μL of MTT (5 mg / mL) in phosphate buffer, continue to incubate at 37 ° C for 4 hours, centrifuge for 5 minutes to remove unconverted MTT, and add 200 μL of dimethyl sulfoxide to each well to dissolve the reduced MTT crystal formazan (form...
Embodiment 3
[0030] Example 3: Cytotoxic activity of compound (A) on HL-60 cells
[0031] HL-60 (human myeloid leukemia cells) were cultured with RPMI 1640 medium containing 10% calf serum, 100 U / mL penicillin and 100 U / mL streptomycin. cells at 1×10 per well 4 Seed into 96-well plates at 37 °C with 5% CO 2 Incubate for 24 hours in a humidified incubator.
[0032] The assay of cell viability uses the improved MTT method, and the specific method is the same as in Example 2. Wherein compound (A) half inhibitory concentration (IC) to HL-60 cell 50 ) obtained from the dose-response curve.
[0033] IC of compound (A) 50 For: 2.08×10 -4 M; while the IC of positive control cisplatin on HL-60 cells 50 is 2.07×10 -5 M.
[0034] Experimental conclusion: This experiment shows that the natural product of erimofen lactone acid has strong cytotoxicity to HL-60 cells, and it may be developed into a new drug with the effect of treating leukemia and related tumors.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com