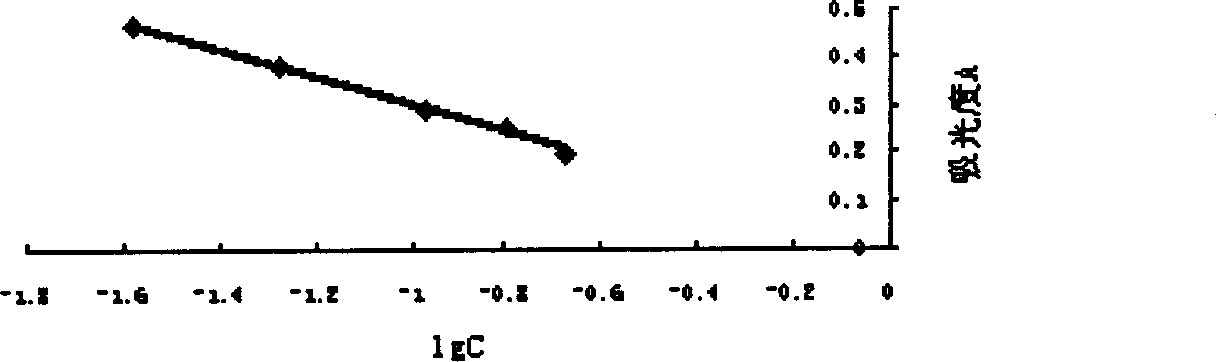Low molecular weight heparin and its preparation method
A low-molecular-weight heparin and average molecular weight technology, applied in the field of low-molecular-weight heparin and its preparation, can solve the problem of low bleeding and achieve the effect of improving the therapeutic index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Embodiment 1, the preparation of low molecular weight heparin
[0072] Add 40g of heparin and 2000ml of distilled water into a 5000ml beaker until the heparin is completely dissolved; at 25°C, add 36% acetic acid to adjust the pH to 3.20. 320 ml of 0.7% sodium nitrite solution was added at one time, and the reaction was stirred at room temperature for 3.0 hours.
[0073] At the end of the reaction, use 4 mol / L sodium hydroxide to terminate the reaction (pH>4.00), and continue to use 4 mol / L sodium hydroxide to adjust the pH=10.0.
[0074] 0.8 g of sodium borohydride was added, and the reaction was stirred at room temperature for 18 hours for reduction. Use hydrochloric acid to adjust pH=4.00 to destroy excess sodium borohydride; then use 4mol / L sodium hydroxide to adjust pH=7.00. Store at less than 10°C. Add 40% v / v of 95% ethanol less than 10°C under the condition of stirring, stir while adding, and stand still under the condition of less th...
Embodiment 2
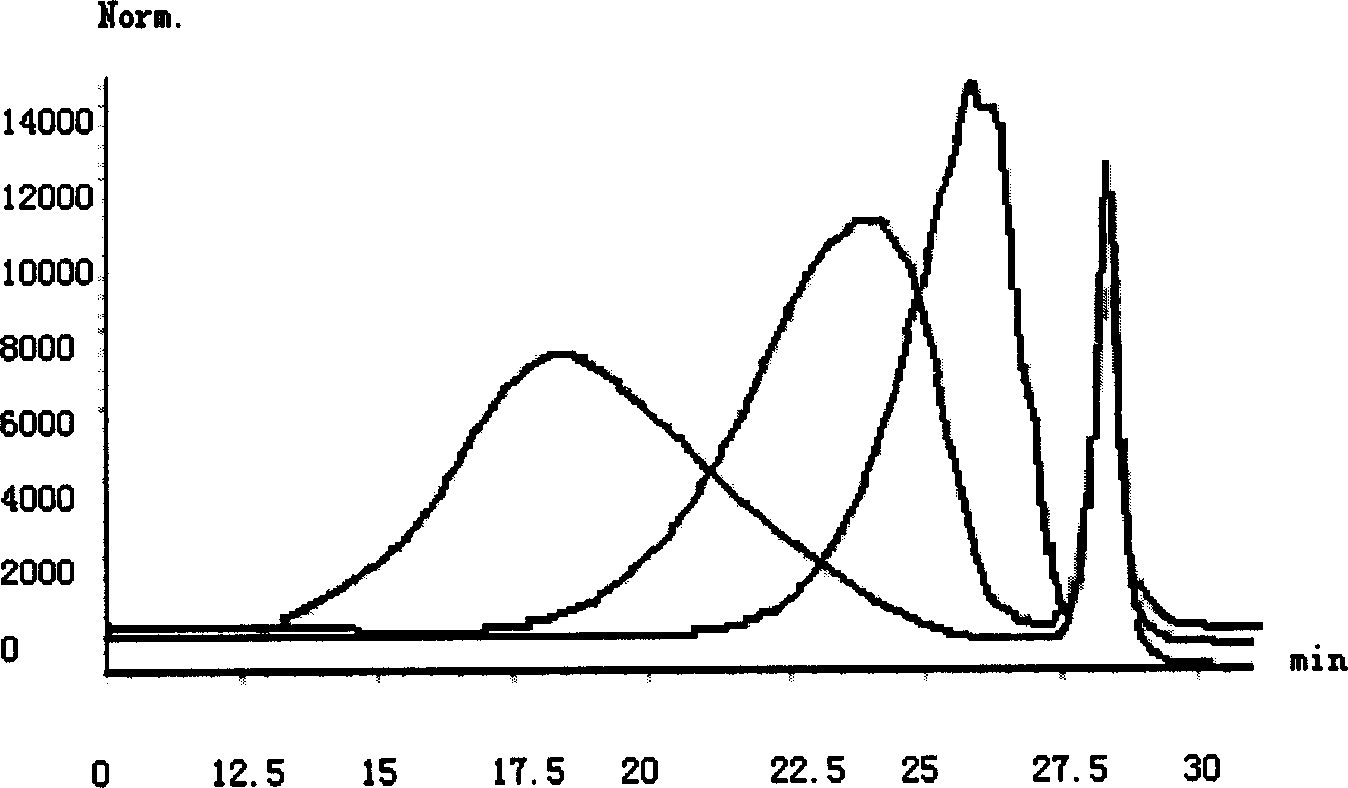
[0077] Embodiment 2, molecular weight analysis of low molecular weight heparin
[0078] The chromatograph selects HP 1100 high-performance liquid chromatography (DAD, RI detector), and the chromatographic column is TSK GELG2000SWXL, 7.8mmID*30cm, 5 μ m, detection wavelength: 234nm, mobile phase: with 2.84% (W / V) sodium sulfate solution Adjust pH to 5.0 with 1mol / L sulfuric acid, column pressure: 35-40kg, flow rate: 0.5ml / min.
[0079] Samples, standard substances and reference substances were prepared into 10mg / ml solution with mobile phase, and injected for analysis.
[0080] (1) System calibration: Inject 25 μl of standard solution, detect using a differential detector (RI) sequentially connected to a UV spectrophotometer with a measuring wavelength of 234 nm, and connect UV to the output end of the chromatographic column, and RI to UV output terminal. The time difference between the two detectors must be accurately measured so that the chromatogra...
Embodiment 3
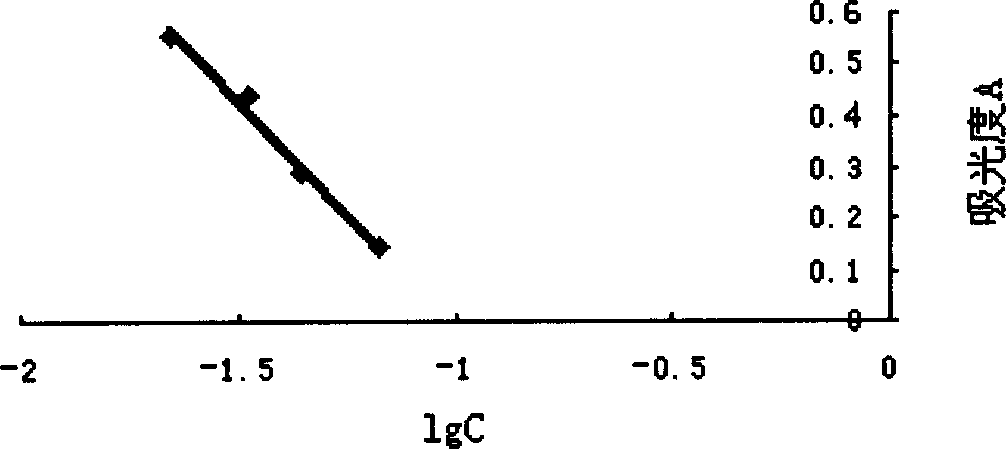
[0085] Embodiment 3, anti-Xa factor potency determination
[0086] Preparation of the standard solution: the standard solution is diluted with tris-hydrochloric acid buffer (pH7.4) to make it contain 0.02, 0.05, 0.1, 0.15, 0.2 anti-factor Xa international units per 1 ml liquid.
[0087] Preparation of sample solution: the low-molecular-weight heparin prepared in Example 1 was dissolved in physiological saline and diluted to 0.1 mg / ml. Dilute 60 times with tris-hydrochloric acid buffer (pH7.4). (The anti-factor Xa titer of the estimated sample is 60IU / mg, and it is prepared into a sample solution containing about 0.1 international units of anti-factor Xa in every 1 milliliter.)
[0088] Take a small test tube, add 50 μl PURIFIED ATIII solution and 50 μl standard solution of each dilution, mix well, incubate in a 37°C incubator for 1 minute, add 100 μl Xa factor solution, mix well, incubate in a 37°C incubator for 1 minute, add 250 μl Chromogenic subs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com