Quality control method for vatility source preparation
A quality control method and preparation technology, which can be used in testing pharmaceutical preparations, measuring devices, pharmaceutical formulations, etc., and can solve problems such as the inability to guarantee the clinical efficacy of Vitality Source preparations, the lack of identification and inspection methods, and the impact on product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the quality control of vitality source tablet
[0042] Identification of Radix Ophiopogon japonicus: take the Vitality Source Tablet of Reference 2, remove the sugar coating, take 1.2g of fine powder, add 40ml of water, add 2ml of sulfuric acid, heat and reflux in a water bath for 1 hour, take it out, let it cool, centrifuge, take the supernatant and pipette Put it into a separatory funnel, extract with ether 3 times, 15ml each time, combine the ether solution, evaporate to dryness, add 1ml of chloroform to the residue to dissolve, and use it as the test solution. In addition, take 1 g of Ophiopogon japonicus reference medicinal material, add 40 ml of water, and start from "adding 3 ml of hydrochloric acid" as above to make a reference medicinal material solution. According to the thin-layer chromatography ("Chinese Pharmacopoeia" 2005 edition, an appendix VI B) test, take 10 μl of each of the above two solutions, respectively spot on the same silica gel G...
Embodiment 2
[0045] Embodiment 2: the quality control of vitality source oral liquid
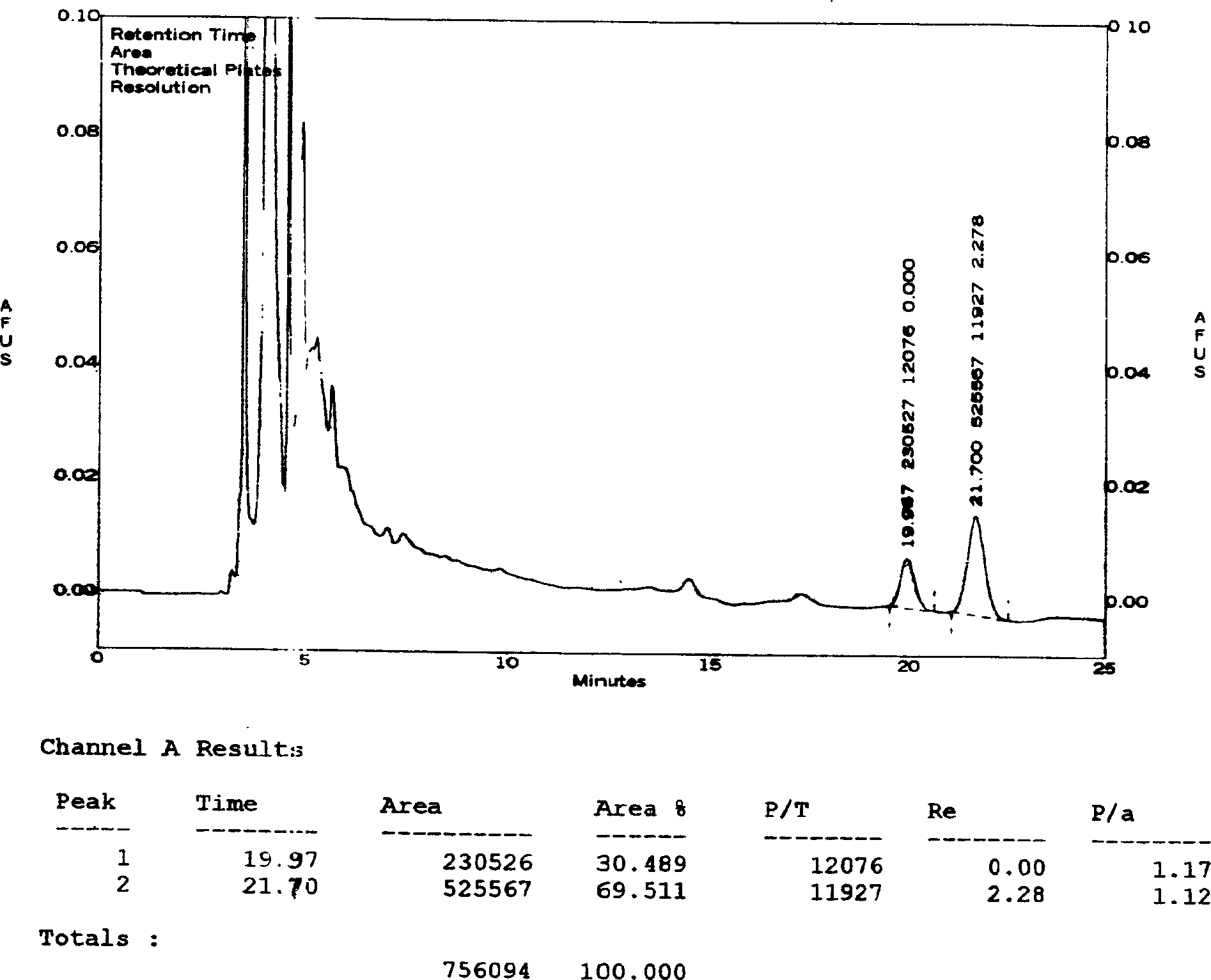
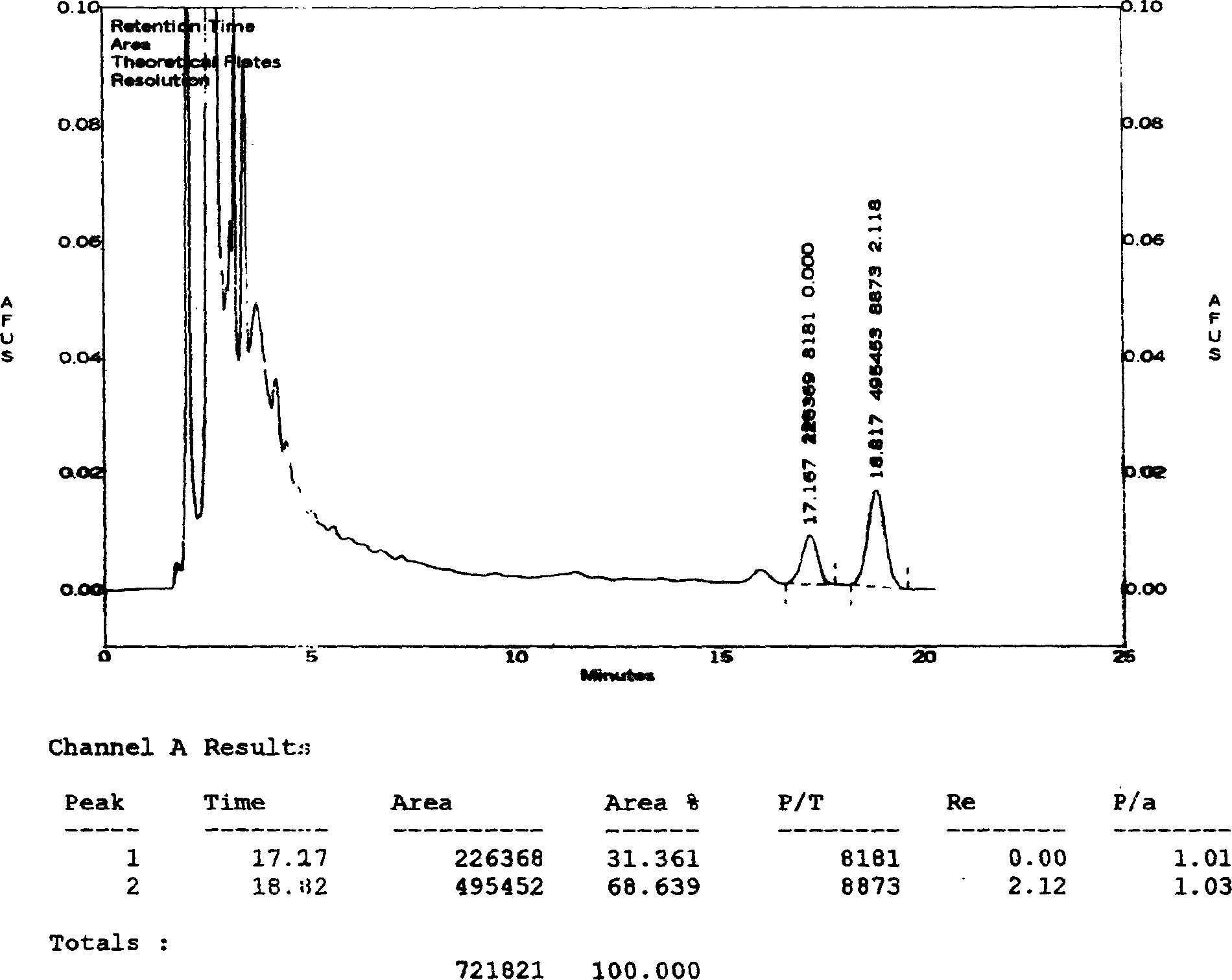
[0046] Determination of the content of ginsenosides:
[0047] Chromatographic conditions and system suitability test: use octylsilane bonded silica gel as filler; acetonitrile-0.08% phosphoric acid (21:79) as mobile phase; detection wavelength 203nm; theoretical plate number according to ginsenoside Rg1, ginsenoside Re peak The calculation should be no less than 2000.
[0048] Preparation of the control solution: Accurately weigh the appropriate amount of reference substances of ginsenoside Rg1 and ginsenoside Re, add methanol to make solutions containing 0.2 mg and 0.4 mg per 1 ml, and obtain.
[0049] Preparation of the test solution: get 50ml of the Vigor Source Oral Liquid of Comparative Document 1, evaporate to dryness, transfer quantitatively to a 100ml conical flask with a stopper with 25ml of chloroform, heat and reflux for 30 minutes, filter, and put the stoppered cone The residue in the bottl...
Embodiment 3
[0052] Embodiment 3: the quality control of vitality source sheet
[0053] (1) Identification of ginsenosides: take the vitality source tablet of reference 2, remove the sugar coating, grind into fine powder, take 3g of fine powder, add 100ml of water, ultrasonically dissolve, filter, take 20ml of filtrate and put it in a separating funnel, and use water-saturated Extract with n-butanol 3 times, 15ml each time, combine the extracts, evaporate to dryness, add methanol 10ml to dissolve the residue, and use it as the test solution. Separately take appropriate amount of ginsenoside Rg1 reference substance and ginsenoside Re reference substance, respectively add methanol to make a solution containing 2mg per 1ml, as the reference substance solution. According to the test of thin-layer chromatography (Appendix VI B of "Chinese Pharmacopoeia" 2005 edition), draw 5 μl of each of the above two solutions, respectively spot on the same silica gel G thin-layer plate, and mix with chlorofo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com