Fingerprint determination method and quality control method of Guyin decoction
A standard fingerprint and fingerprint technology, which is applied in the fingerprint determination method and quality control field of Guyin decoction substance reference, can solve the problems of inability to effectively control the production process and product quality, and inability to better guarantee the clinical efficacy, etc. Reduce the possibility of manual processing, ensure stability and safety of use, and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
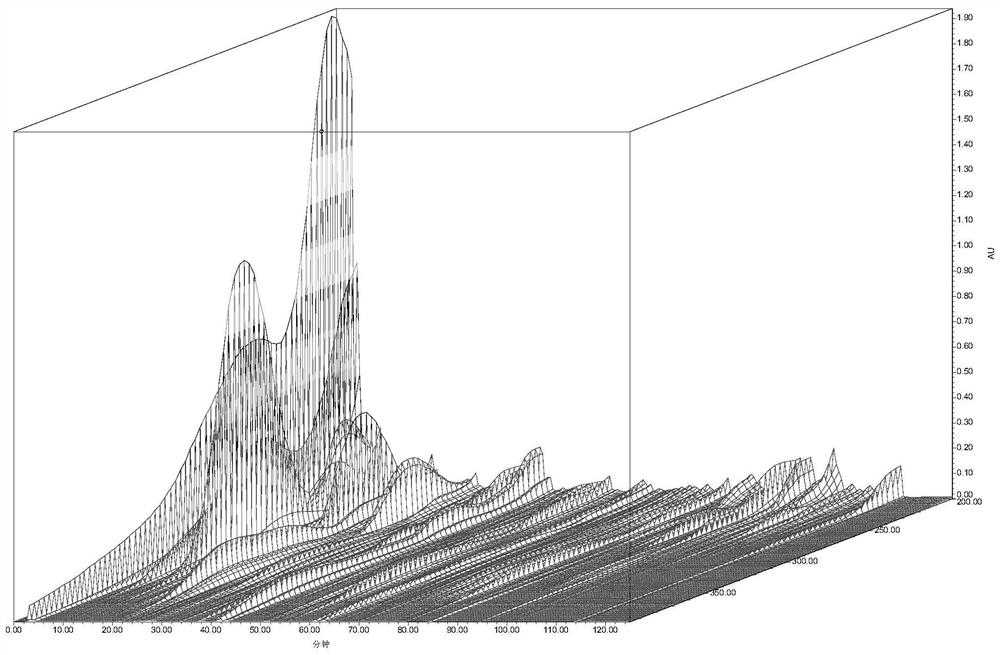
Image
Examples
Embodiment 1
[0062] Embodiment 1 Fingerprint determination method of Guyin decoction material benchmark
[0063] 1.1 Instruments and reagents
[0064] Instruments: Waters 2695-2996 high performance liquid chromatograph (Waters, USA); SHIMADZU (Shimadzu) AUW120D 1 / 100,000 electronic balance (Shimadzu, Japan), BSA124S 1 / 10,000 electronic balance (Sartorius, Germany).
[0065] Reagents: 5-Hydroxymethylfurfural (batch number: 111626-201711, purity 90.8%), morroniside (batch number: 111998-201602, purity 96.3%), chlorogenic acid (batch number: 110753-201817, purity 96.3%) 96.8%), loganin (lot number: 111640-201707, 99.2% purity), hypericin (lot number: 111521-201004, 93.9% purity), 3,6'-di-sucrose (lot number: 111848) -201604, 96.7% purity, China National Institute for Food and Drug Control), ammonium glycyrrhizinate (batch number: 110731-201720, 97.7% purity), Schisandrin A (batch number: 110857-201714, 99.9% purity) reference substances were all Purchased from China National Institute for F...
Embodiment 2
[0135] The quality control method of embodiment 2 Guyinjian standard granules
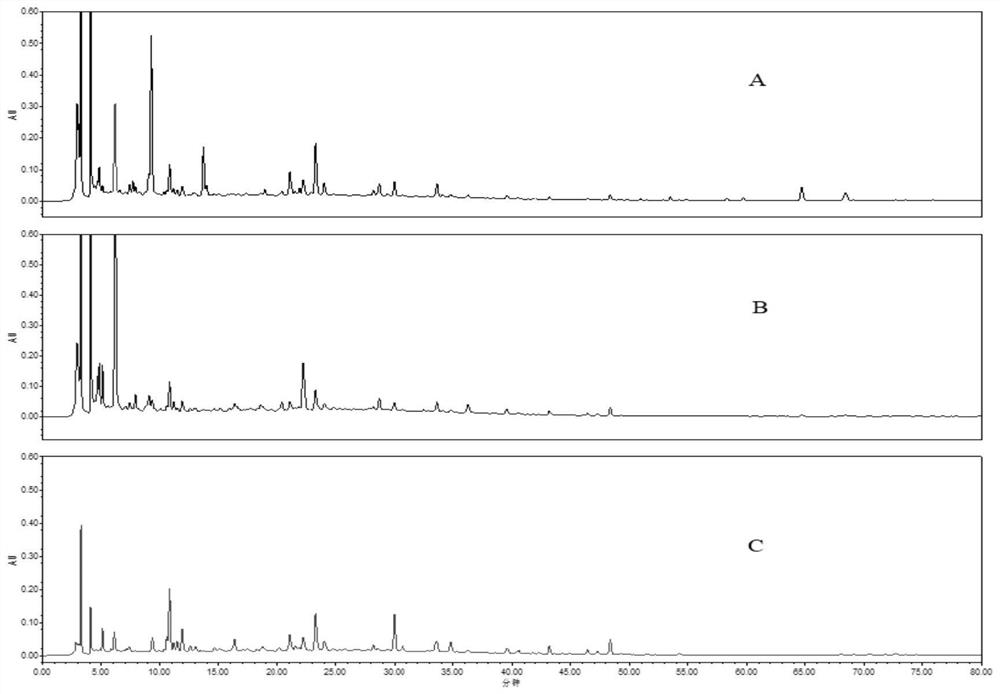
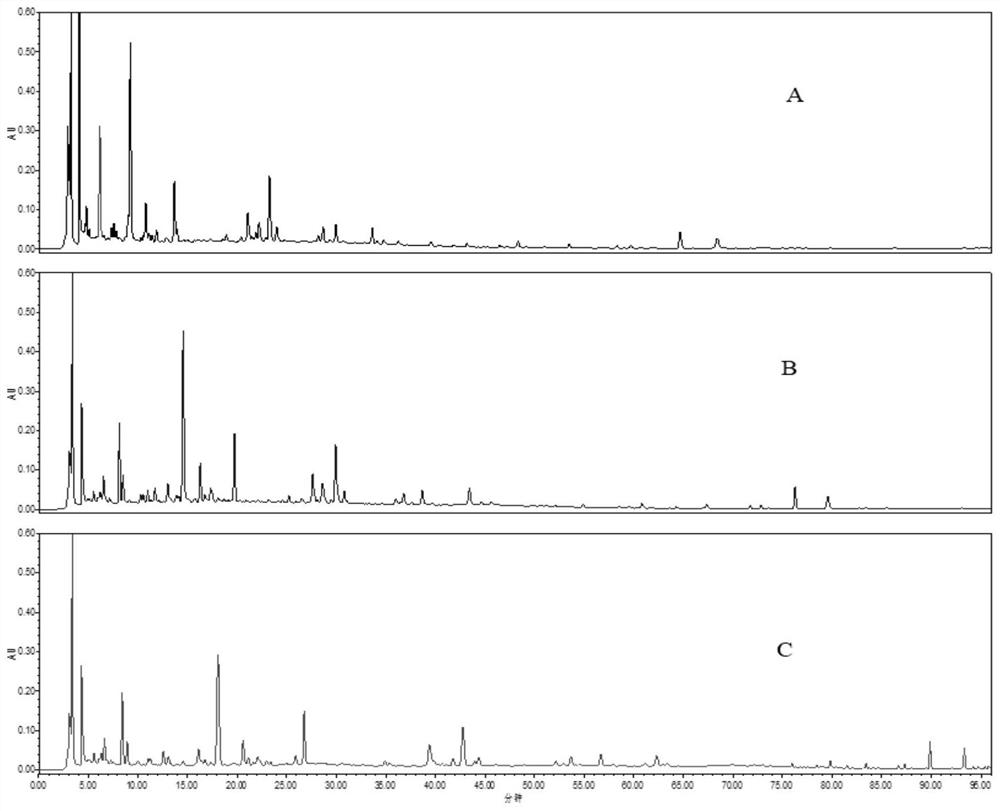
[0136] (1) according to the method in 1.2.1(1) in Example 1, prepare the standard fingerprint of Guyin decoction substance benchmark, adopt the assay method in Example 1 to establish the standard fingerprint of Guyin decoction substance benchmark;
[0137] (2) get Guyinjian standard granule need testing solution, obtain fingerprint according to the assay method in embodiment 1;
[0138] (3) Comparing the fingerprint obtained in step (2) with the standard fingerprint obtained in step (1), if the product conforms, it is a qualified product, and if it does not conform, it is an unqualified product.
[0139] The requirements to be met include: the fingerprint of the test sample should present 12 characteristic peaks, of which 8 peaks should have the same retention time as the corresponding reference peaks in the standard fingerprint; peaks corresponding to the hyperin reference It is an S peak, and th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com