Hydroxycamptothecine injection and its prepn process
A technology for hydroxycamptothecin and alkali injection, which can be used in pharmaceutical formulations, drug combinations, antitumor drugs and other directions, can solve the problems of affecting the curative effect, inaccurate content determination, increased toxicity, etc., and achieves a simple and feasible preparation process. The effect of strong control and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] R x1 50ml: 10mg
[0032] Hydroxycamptothecin 0.10g
[0033] L-Arginine 0.390g
[0035] Hydrochloric acid (1mol / L) appropriate amount
[0036] Add water for injection to 500ml
[0037] Preparation process: Take L-arginine and add 250ml of water for injection to dissolve, add hydroxycamptothecin, stir, add sodium chloride after the solution is clear, stir and mix well, adjust the pH to about 8.3 with hydrochloric acid, then add water for injection to 500ml , measure the content and pH value, filter through a 0.45 μm microporous membrane after passing the test, potting, and autoclave at 115° C. for 30 minutes to obtain the product.
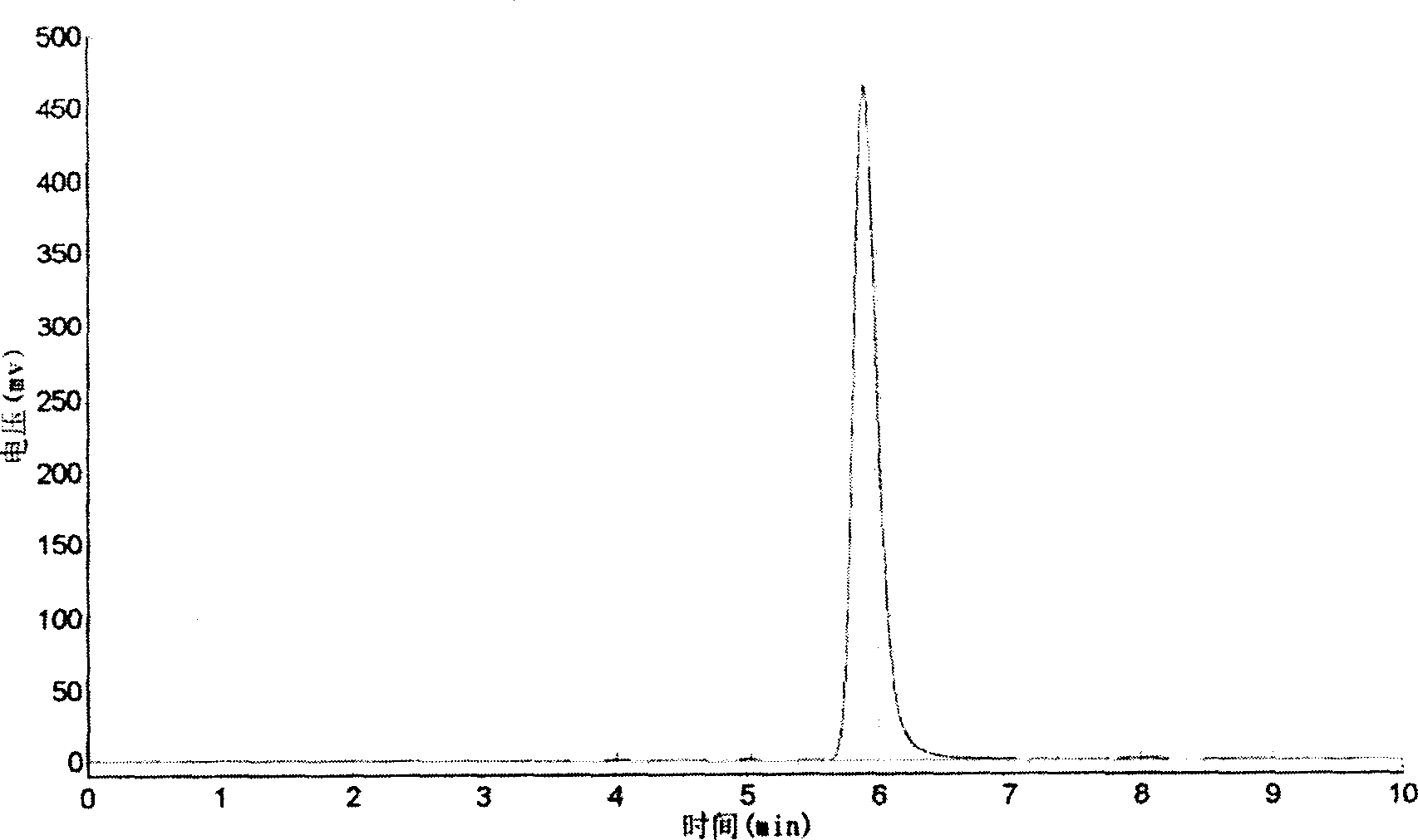
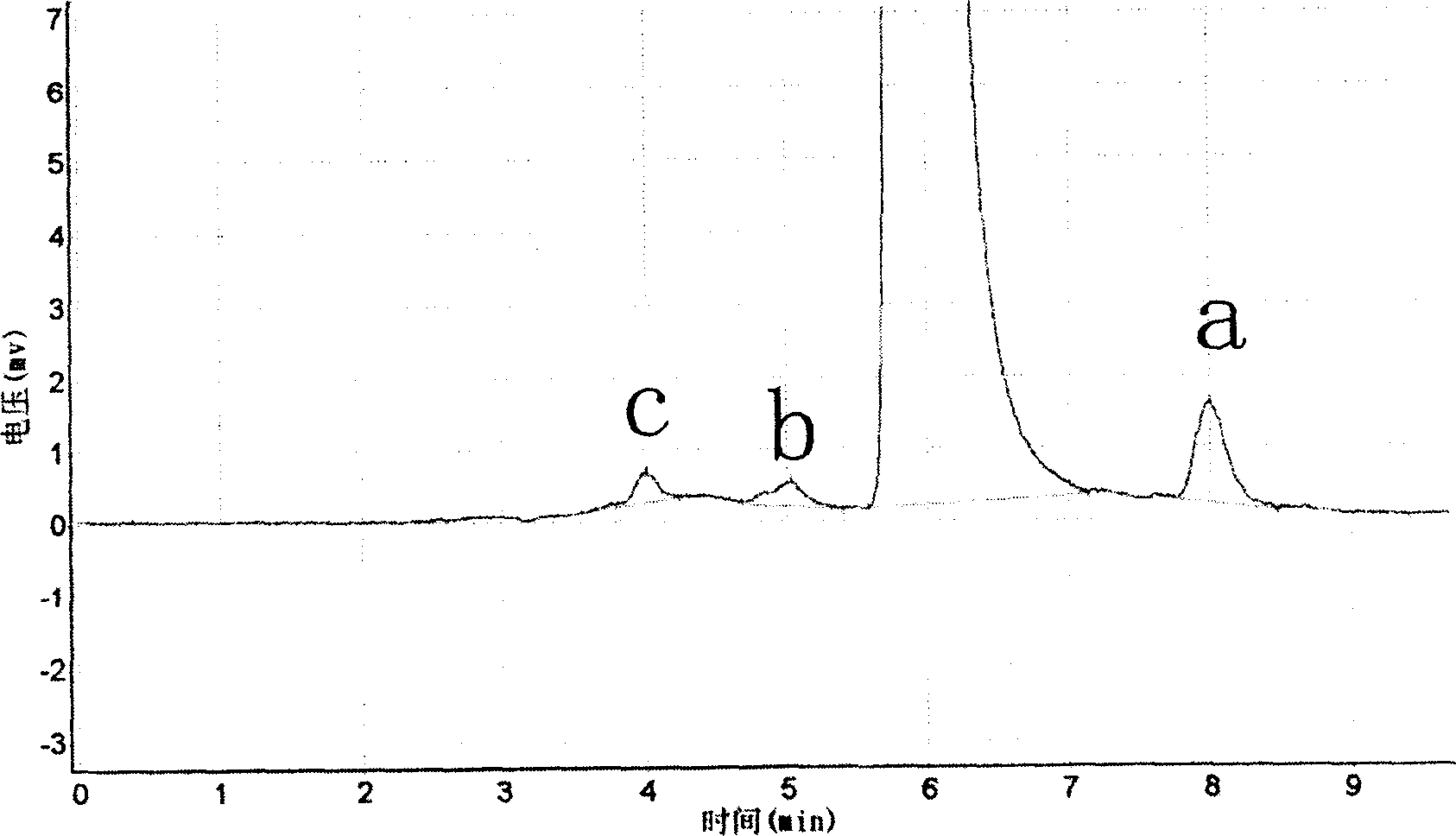
[0038] Detection method::
[0039] Instrument: (Shimadzu) LC-10A liquid chromatograph
[0040] Chromatographic column ALTEX ULTRSPHERE-ODS (4.6×150mm, 5um)
[0041] The mobile phase is methanol-0.4% phosphoric acid aqueous solution (57:43)
[0042] Detection wavelength 342nm Column temperature 40℃ Flow ...
Embodiment 2
[0045] R x1 100ml: 9mg
[0046] Hydroxycamptothecin 0.09g
[0047] L-Arginine 0.43g
[0049] Hydrochloric acid (1mol / L) appropriate amount
[0050] Add water for injection to 1000ml
[0051] The preparation process is the same as in Example 1.
Embodiment 3
[0053] R x1 250ml: 11mg
[0054] Hydroxycamptothecin 0.11g
[0055] L-Arginine 0.47g
[0056] Sodium chloride 22.48g
[0057] Hydrochloric acid (1mol / L) appropriate amount
[0058] Add water for injection to 2500ml
[0059] The preparation process is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com