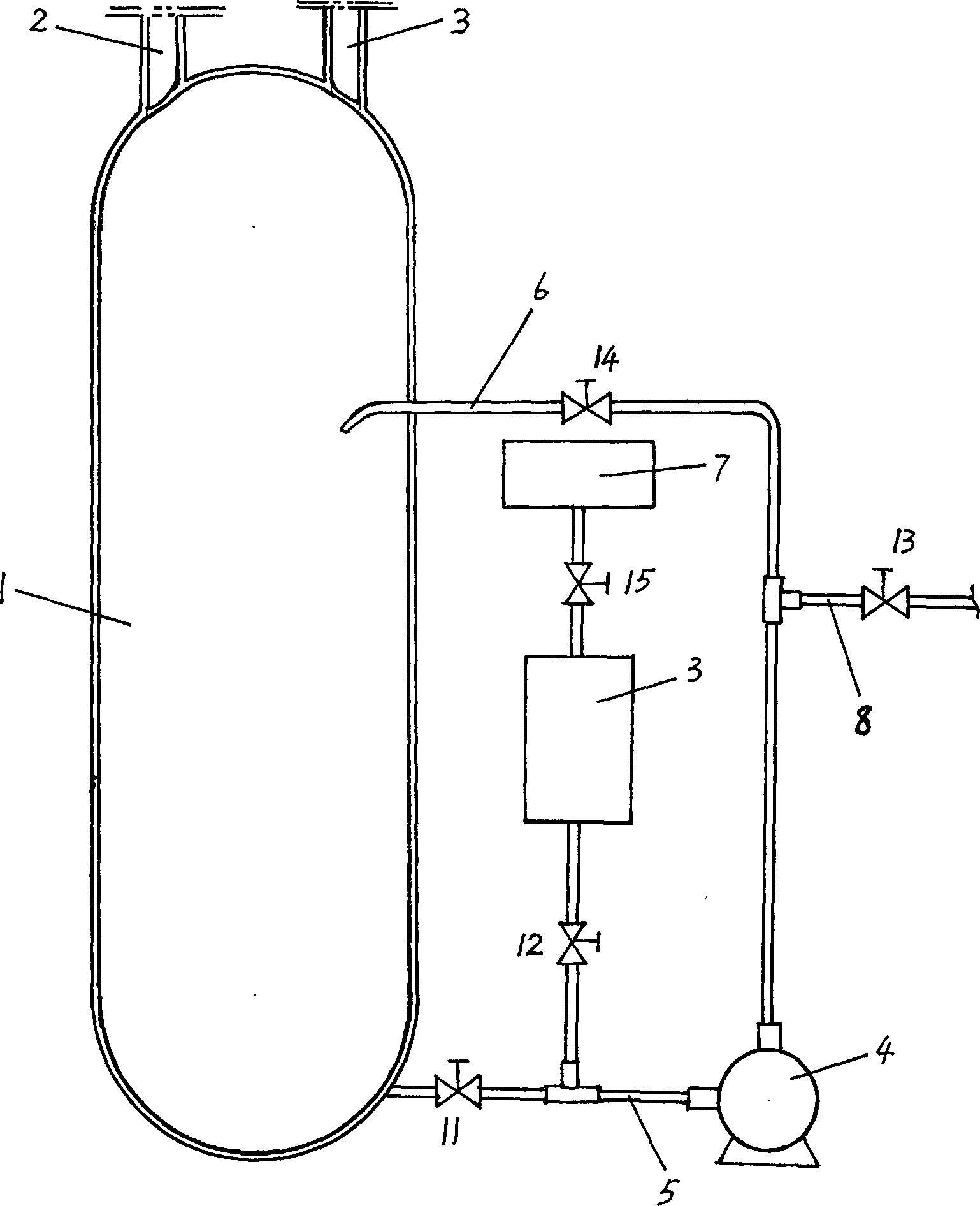
Preparation method of 1,1-difluoro ethane and its production equipment
A difluoroethane and production equipment technology, applied in the field of 1.1 difluoroethane preparation, can solve the problems of increasing unit consumption, increasing high-boiling by-products, rapid catalyst failure, etc., to reduce unit consumption and reduce long-term The diameter ratio and the effect of reducing equipment material consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment one, catalyst service life comparison:
[0033] Reaction device: the diameter of the reaction kettle is 600 mm, and the height is 2000 mm. In this embodiment, the external circulation system is activated.
[0034] Reaction conditions: temperature 22-32°C, choose 28°C in this example, pressure 0.03-0.3mpa, choose 0.05mpa in this example (both are controlled by hand), the initial liquid level of hydrofluoric acid is 60-70% of the volume of the reactor %, 60% is selected in this example, and the feeding rate of acetylene is 0.08-0.12 kg / hour per kilogram of hydrofluoric acid plus acetylene, which is converted into volume. In this example, acetylene 10M is selected 3 / hour, the reaction should be carried out continuously, and stop the reaction when the conversion rate (chromatographic analysis) drops to 60%, draw conclusions as shown in table 1:
[0035] Reaction temperature (°C)
Embodiment 2
[0036] Embodiment 2, comparison of unit consumption of materials:
[0037] Reaction device: with embodiment one;
[0038] Reaction condition: with embodiment one;
[0039] The conclusions are shown in Table 2:
[0040] Reaction temperature (°C)
Embodiment 3
[0041] Embodiment three, catalyst adds comparative test:
[0042] Reaction device: with embodiment one;
[0043]Reaction condition: with embodiment one;
[0044] The first group of reactions is not to discharge the raffinate, do not add catalytic Liu, and the second group of reactions discharges 1 / 3 of the raffinate (indicated by the liquid level meter) in the reactor when the conversion rate is as low as 60%, and adds an equal amount of Catalyst, its result is as shown in table 3:
[0045] Reaction temperature (°C)
[0046] From the above experiments, it can be concluded that the present invention has the advantages of reducing raw material unit consumption, prolonging reaction time and improving reaction efficiency compared with existing devices and techniques.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com