Positive electrode material for lithium ion cell and synthesizing method thereof
A technology for lithium ion batteries and positive electrode materials, applied in battery electrodes, lithium storage batteries, positive electrodes, etc., can solve problems such as unfavorable industrial applications, and achieve the effects of easy industrial scale production, complete crystal structure, and convenient and easy-to-obtain raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis of cathode material LiNi 0.8 co 0.2 o 2
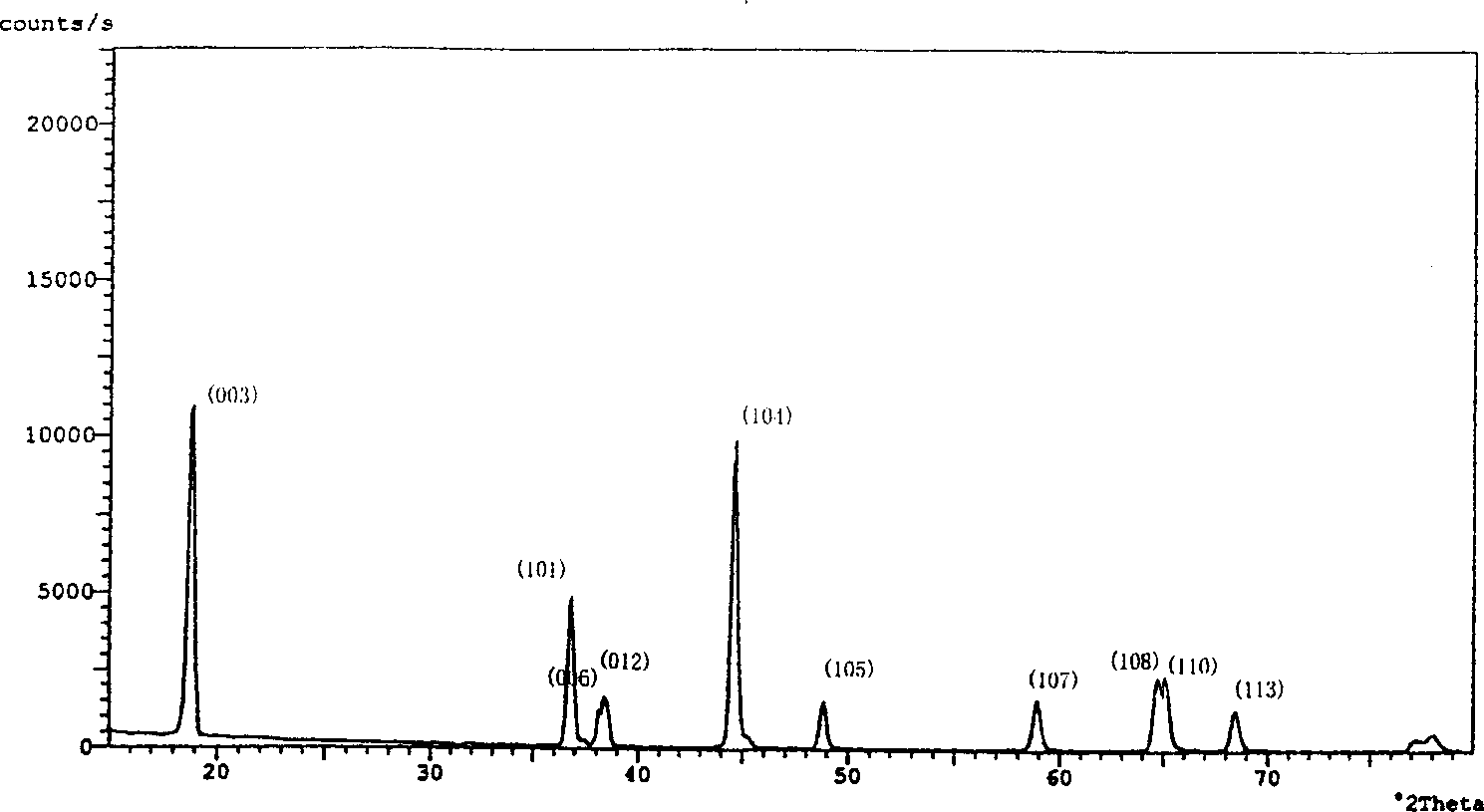
[0027] 0.58mol of LiOH·H 2 O(AR) and 0.5mol LiNO 3 (AR) was placed in an agate mortar and ground carefully, and 0.8mol of Ni(OH) 2 (Containing Ni is 61.35%) and 0.2mol of Co 2 o 3 (CA) Grind evenly in an agate mortar, then mix the two mixtures together and grind evenly to form a mixed powder. Baking the mixed powder in an air environment of 630°C for 7 hours, taking it out and cooling to room temperature, grinding the sintered mixture into powder particles, and then returning to the furnace at a heating rate of 250°C / hour to about 750°C and roasting for 15 hours , while passing oxygen, the flow rate of oxygen is kept stable and controlled at 25ml / min. After the calcination, it is naturally cooled to below 450°C with the furnace to stop the flow of oxygen. After continuing to cool to room temperature, it is taken out for grinding. Analytical characterization and electrochemical test results such as figure 1 , ...
Embodiment 2
[0033] Synthesis of cadmium-containing lithium nickel cobalt oxide cathode material Li x Ni 0.8-y co 0.2 Cd y o p
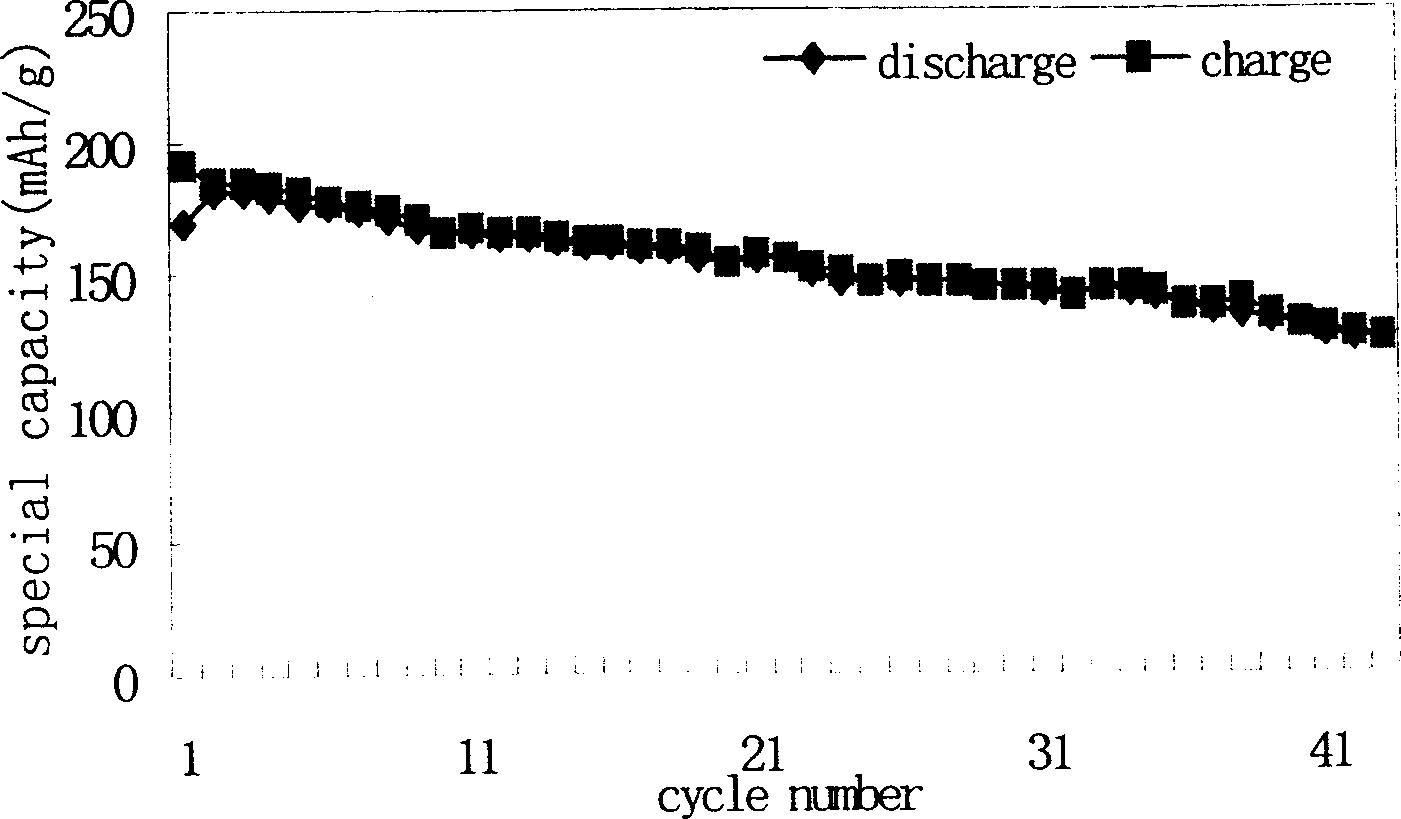
[0034] With Cd-containing spherical Ni(OH) 2 (Containing Ni is 59.45%, Cd is 3.15%) to replace Ni(OH) in Example 1 2 , other process conditions are consistent with embodiment 1. The chemical composition of the synthesized material is analyzed by ICP-AES, and the actual metering formula of the measured material is: Li 1.06 Ni 0.74 co 0.19 Cd 0.02 o 2.04 . Other analytical characterization and electrochemical test results such as Figure 4 , 5 and 6, and related data are listed in Table-2.
[0035] Table 2:
[0036] I 003 / I 104 : 1.40 Initial discharge specific capacity: 157.8mAh / g
[0037] Particle size: 200~700nm First Coulombic efficiency: 87.1%
[0038] Capacity retention rate: 91%
Embodiment 3
[0040] Synthesis of zinc-containing lithium-nickel-cobalt composite oxide cathode material LixNi 0.8-y co 0.2 Zn y o p
[0041] 0.65mol of LiOH·H 2 O(AR) and 0.5mol LiNO 3 (AR) Grind finely in an agate mortar. In addition, 0.8mol of high-density Zn-containing Ni(OH) 2 (containing 60.03% Ni, 4.14% Zn) and 0.2mol of Co 2 o 3 (CA) Ball milled in a planetary ball mill for 3 hours to make it evenly mixed. Then mix the two mixtures together and grind until smooth. Roast the uniformly ground mixture for 6 hours at 650°C in an air environment, take it out and cool it to room temperature, grind the sintered mixture into powder particles, and then return to the furnace to increase the temperature to 750°C at a rate of 250°C / hour Left and right roasting for 16 hours, while passing oxygen, the oxygen flow rate is kept stable and controlled at 15ml / min. After the calcination is finished, use program control to lower the temperature to below 500°C, stop the oxygen flow, continue ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Coulombic efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com