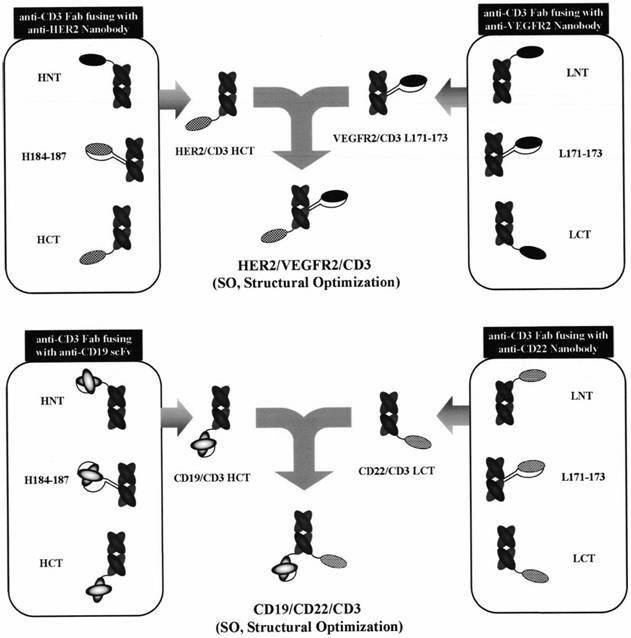
Preparation method of tri-specific antibody based on structure optimization protein activity
A protein activity and specificity technology, applied in the direction of antibodies, hybrid immunoglobulins, anti-tumor drugs, etc., can solve problems such as limiting the clinical application of bispecific antibodies, reduce the number of administrations and doses, and simplify expression and purification. Long half-life effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] Example 1 Construction and eukaryotic expression of bi / trispecific antibodies
[0146] 1.1 Vector construction
[0147] Construction of CD3-HC-HER2-VHH and CD3-LC expression vectors:
[0148] The heavy chain of the HER2 / CD3 bispecific antibody is linked by CD3-HC (SP34) and HER2-VHH, respectively, in the following manner: HER2-VHH is linked to the CD3-HC variable region (VH) structure by a rigid linker peptide HE Linker Domain N-terminal 1E; or HER2-VHH is linked to the CD3-HC variable region (VH) domain N-terminal 1E by a rigid linker peptide PD Linker; or HER2-VHH is chimeric to CD3 by a coiled coil linker peptide Coiled Coil Linker - Between 184S-187L of the HC constant region (CH1) domain, while deleting 185S and 186G; or connecting HER2-VHH to the C-terminal 228C of the CD3-HC constant region (CH1) domain through a flexible linker (G4S) 3 Linker ; Light chain CD3-LC without any modification. The coding genes of CD3-HC-HER2-VHH and CD3-LC were synthesized accordi...
Embodiment 2 3
[0176] Example 2 Purification of trispecific antibodies
[0177] 2.1 Protein G affinity purification
[0178] Take 400 mL of the cell supernatant from 1.2 in Example 1 above, centrifuge at 15,000 rpm for 30 min at 4°C, collect the supernatant, filter with a 0.45 μm filter, and place it on ice for later use. Take 4 mL of Protein G (20% ethanol / Protein G 1:1) into the column, rinse with Binding buffer three times, and press the resin surface with a gasket. Equilibrate the Protein G column with 20 mL of Binding buffer. Every 10 mL was loaded, and the sample was passed through the Protein G column at a constant speed (about 0.5 mL / min). Wash the Protein G column with 40 mL of Binding buffer at a constant speed (about 1 mL / min). First, add 10% of the eluate volume of neutralization buffer to the collection tube of the elution tube, add Elution buffer to the column, and elute once with 5 mL until the protein concentration cannot be quantified. The collected protein samples were ...
Embodiment 3
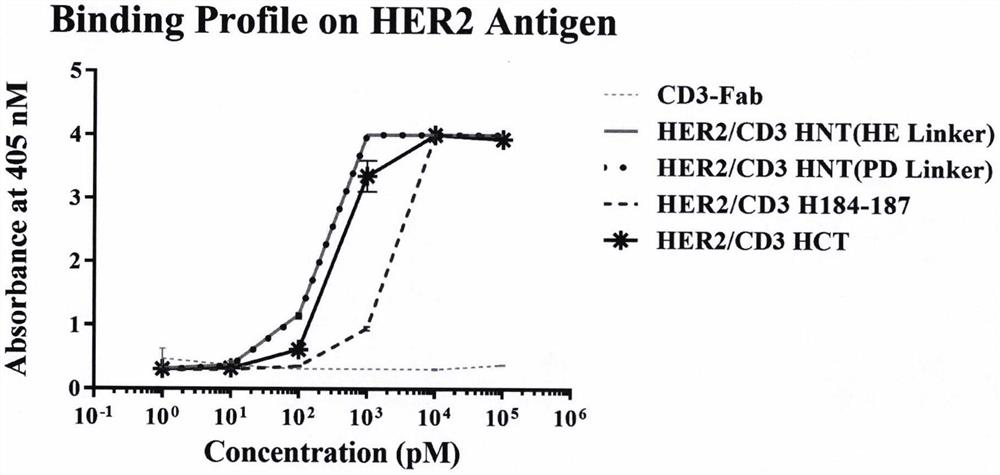
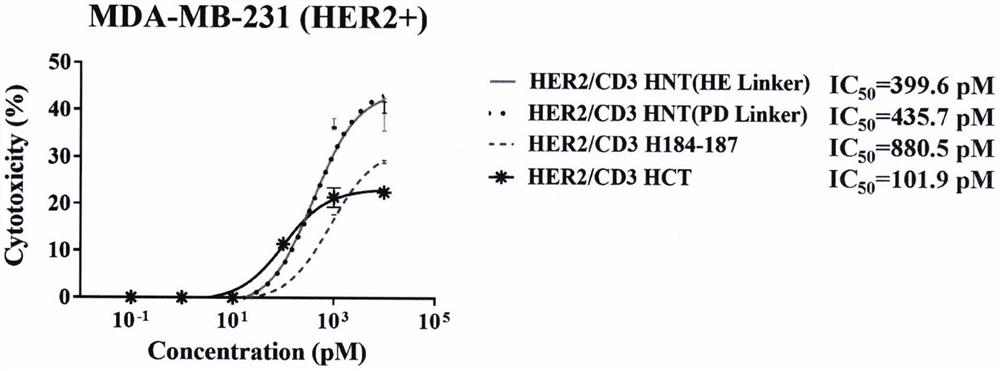
[0179] Example 3 Biological function evaluation of HER2 / VEGFR2 / CD3 trispecific antibody
[0180] 3.1 Antigen binding validation of HER2 / CD3 bispecific antibodies with different structures
[0181] The HER2 ECD-Fc antigen protein was diluted with PBS to 10 μg / mL and coated in a 96-well ELISA plate, 100 μL / well, and coated overnight at 4°C. Discard the liquid in the plate, wash the plate twice with PBST, add 200 μL / well of PBST solution of 5% skim milk, block at room temperature for 2 hours, and drain the blocking solution in the well. The bispecific antibodies to be tested HER2 / CD3 HNT (HE Linker), HER2 / CD3 HNT (PDLinker), HER2 / CD3 H184-187, HER2 / CD3 HCT were diluted 10 times with blocking solution for 8 gradients, and the initial antibody concentration was 100 nM, 3 duplicate wells for each concentration gradient, 100 μL / well was added to the well plate, and incubated at room temperature for 2 h. The plate was washed three times with PBST and then drained. The HRP-labeled an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com