Recombinant chimpanzee source adenovirus for expressing rabies virus G protein and preparation method of recombinant chimpanzee source adenovirus
A rabies virus and chimpanzee technology, applied in the field of recombinant chimpanzee-derived adenovirus and its preparation, can solve problems such as no reported rabies virus vaccine, achieve excellent immune protection effect, efficiently induce immune response, and produce safe and convenient effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] This embodiment provides a method for constructing an E1-deleted replication-deficient adenoviral vector pAdC68 (pAdC68-E1-deleted), which specifically includes the following steps:
[0067] (1) Construction of plasmid pNEB193-KE
[0068] According to the multiple cloning site on the pNEB193 vector and the sequence of the adenovirus AdC68 genome, the primers for amplifying the KE fragment on the adenovirus AdC68 genome (refer to Table 1) were designed, and the target product KE fragment was amplified by PCR. The total volume of the PCR amplification system was 50 μL, and the reaction cycle parameters were: pre-denaturation at 95°C for 5 min; denaturation at 94°C for 1 min; annealing temperature at 57°C for 30 s; extension at 72°C for 2.2 min. The KE fragment amplified by EcoRI and KpnI double enzyme digestion PCR, the KE fragment was purified by agarose gel, connected to the pNEB193 vector that had been digested by the same enzyme, transformed into DH5α competent cells,...
Embodiment 2
[0085] In this embodiment, the G protein-encoding gene Gp gene of rabies virus and the WPRE regulatory element are linked into pAdC68-E1-deleted in Example 1.
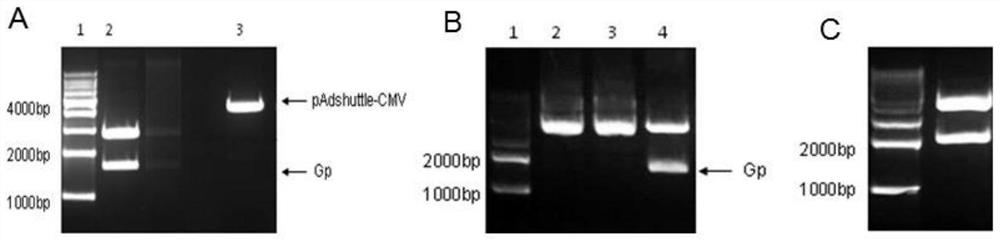
[0086] (1) According to the nucleic acid sequence of the Gp coding region in the rabies virus ERA strain in GenBank, the codon-optimized Gp gene (as shown in SEQ ID NO.1) was synthesized, and the WPRE sequence was inserted at the 3', and the optimized The 5' end of the target gene of the codon is inserted into EcoRI and NheI restriction sites in turn, and the 3' end is connected to the XbaI restriction site ( figure 1 ), the optimized Gp gene was cloned into the commercially available vector pUC57 vector by double digestion with EcoRI and XbaI to obtain the recombinant plasmid pUC57-0 / Gp-WPRE.
[0087] (2) The pUC57-0 / Gp-WPRE plasmid was digested with NheI and XbaI to form the target gene with cohesive ends, and the adenovirus shuttle vector pShuttle-CMV was digested with NheI and XbaI to obtain a linearized vector , ...
Embodiment 3
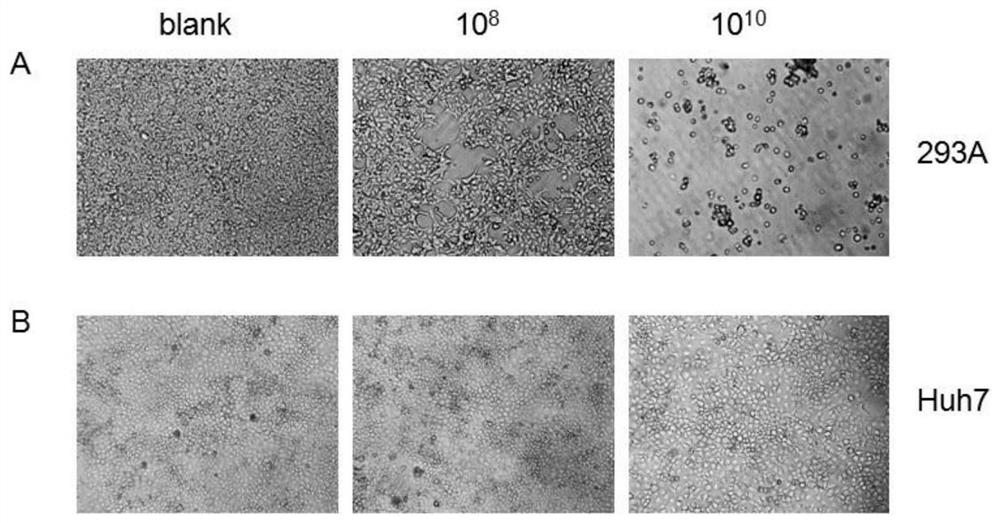
[0090] The recombinant adenoviral plasmid pAdC68-Gp-WPRE in Example 2 was linearized with the restriction endonuclease PacI, and the plasmid was transferred into HEK293 cells (6-well plate) by lipofection, and incubated at 37°C for 6- On day 8, obvious plaques appeared. After the cells became round and suspended, the cells were collected, and after repeated freezing and thawing three times, the virus supernatant was taken to infect HEK293 cells (75ml cell culture flask). Repeat the above steps until an appropriate amount of virus is collected (about 20-40 150ml cell culture flasks), purify the virus by cesium chloride density gradient centrifugation, measure the OD value, add glycerol with a final concentration of 10% and store at -80°C. Extract viral genomic DNA (virus content 10 12 vp), and BglII, BamHI, NheI restriction map analysis. Use the CMV promoter universal primer to detect the nucleic acid sequence of the target fragment. The purified virus was passed continuousl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com