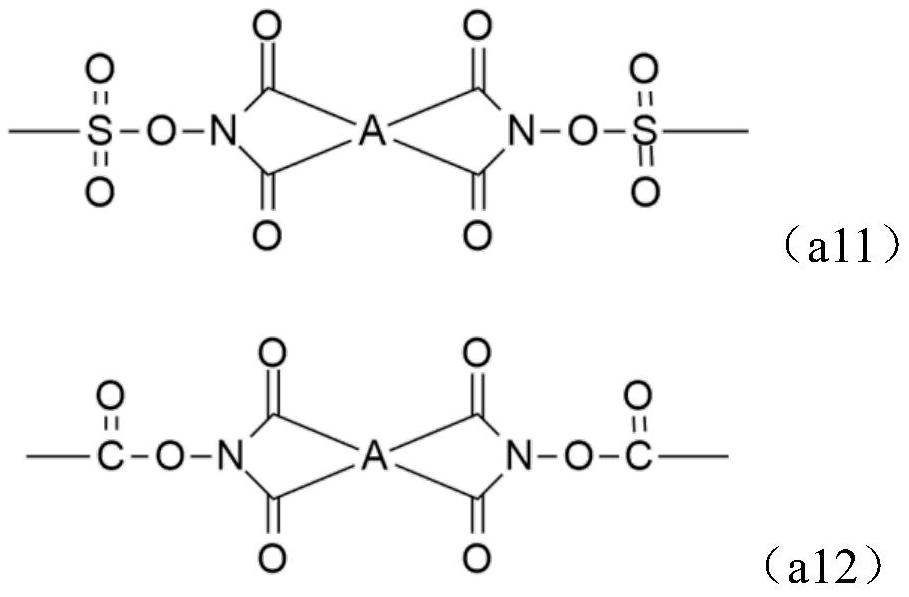
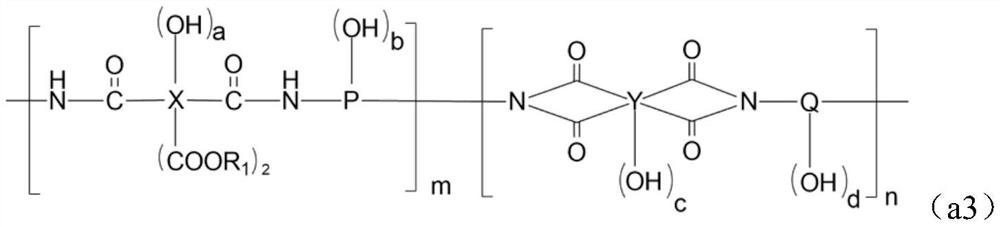
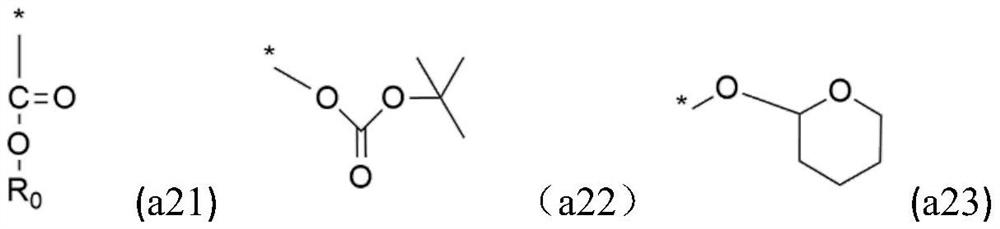Alkali-soluble resin, positive photosensitive resin composition, cured film and display device
A positive-type photosensitive resin and alkali-soluble resin technology, applied in the field of positive-type photosensitive resin compositions, cured films, display devices, and alkali-soluble resins, can solve the problems of slow development, loss of dark film, poor contrast or image resolution, etc. , to achieve high exposure sensitivity and improve the effect of dissolution rate ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0127] The above and other advantages of the present invention can be better understood through the following examples, which are not intended to limit the scope of the present invention.
[0128] Abbreviations:
[0129] ODA: 4,4'-diaminodiphenyl ether
[0130] 6FAP: 2,2-bis(3-amino-4-hydroxyphenyl)hexafluoropropane
[0131] SiDA: 1,3-bis(3-aminopropyl)-1,1,3,3-tetramethyldisiloxane
[0132] 6FODA: 2,2'-bis(trifluoromethyl)-4,4'-diaminodiphenyl ether
[0133] 6FDA: 2,2'-bis(3,4-dicarboxylic acid phenyl)hexafluoropropane dianhydride
[0134] ODPA: 3,3',4,4'-diphenyl ether tetracarboxylic dianhydride
[0135] BTDA: 3,3',4,4'-benzophenonetetracarboxylic dianhydride
[0136] CBDA: Cyclobutanetetracarboxylic dianhydride
Synthetic example 1
[0138] Synthesis of diamine derivative A1 of 6FDA
[0139] Dissolve 0.1 mol of 2-amino-4-nitrophenol in 50 ml of acetone and 0.34 mol of propylene oxide, cool down to -15°C, and slowly dropwise add the A3 solution (0.048 mol, dissolved in 150 ml of propylene glycol) synthesized in Synthesis Example 3. In monomethyl ether), the reaction was continued at -15°C for 4h after dropping, then warmed to room temperature, filtered, the filter cake was washed with acetone for several times and then vacuum-dried at 50°C for 10h to obtain the nitro intermediate.
[0140] The nitro compound was dissolved in 100 ml of NMP, 3 g of 5% palladium-carbon was added, hydrogen was introduced into the autoclave, the room temperature was continuously stirred for 6 h, then the reaction solution was filtered to remove the catalyst, the filtrate was poured into water, and a solid product was precipitated. Filtration and vacuum drying at 50°C for 24h gave the diamine derivative A1 of 6FDA.
[0141]
Synthetic example 2
[0143] Synthesis of diamine derivative A2 of 6FDA
[0144] The only difference from Synthesis Example 1 is that the raw material A3 is replaced with A4 synthesized in Synthesis Example 4, and other conditions remain unchanged to obtain the diamine derivative A2 of 6FDA.
[0145]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com