Preparation method of metaraminol bitartrate
A technology of metaraminol bitartrate and tartaric acid, which is applied in the field of preparation of metaraminol tartrate, can solve the problems of high cost of chiral resolution and no chiral selectivity of metaraminol bitartrate, so as to reduce the cost of splitting and avoid splitting Good separation of isomers and chiral selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
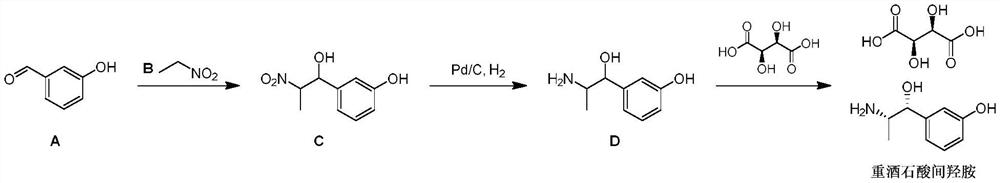
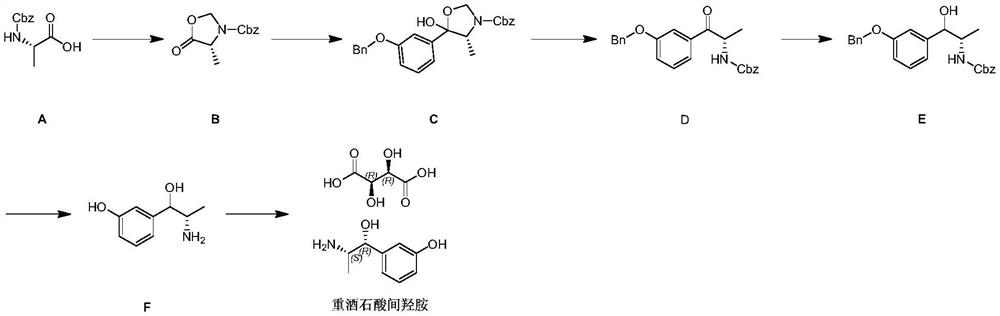
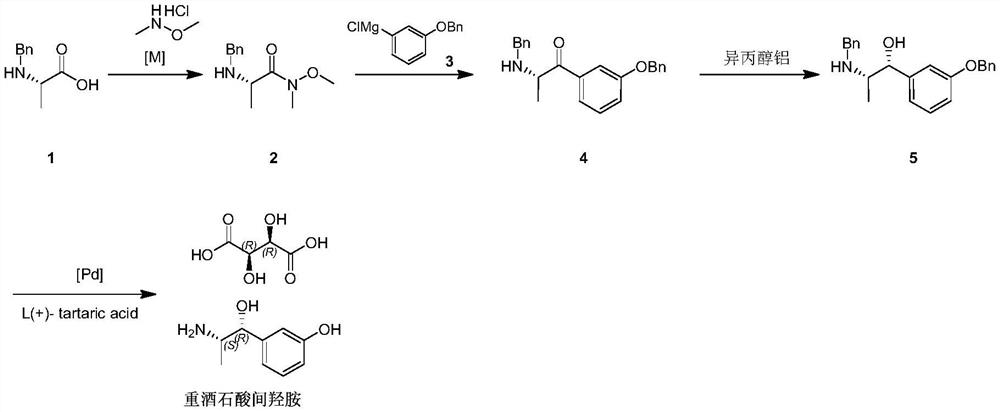
[0026] A kind of preparation method of metaraminol tartrate disclosed by the invention, its synthetic route is as follows:
[0027]
[0028] Where [M] is a conventional organic basic reagent, such as triethylamine, DBU, DIPEA, etc.; [Pd] is a palladium catalyst, such as palladium carbon, palladium hydroxide carbon. Specific steps are as follows:
[0029] S1: Take the structural formula as The compound 1 is reacted with N,O-dimethylhydroxylamine hydrochloride in a solvent, and a base is added during the reaction to obtain the structural formula: Compound 2.
[0030] The preparation steps of compound 2 are as follows: add 100ml DCM to the reaction flask, then add 10g compound 1, stir and cool down to 0-10°C, then dropwise add 10g liquid triethylamine, add 6g N,O- dimethyl hydroxylamine salt acid salt, keep warm at 0-10°C, stir for 4 hours; add 300ml 1mol / L hydrochloric acid solution dropwise after the controlled reaction in TLC (thin-layer chromatography) to adjust pH≈1,...
Embodiment 2
[0038] The difference between the preparation method of metaraminol tartrate disclosed in this example and Example 1 is that the solvent uses toluene or dioxane.
Embodiment 3
[0040] The difference between the preparation method of metaraminol bitartrate disclosed in this example and Example 1 is that DBU or DIPEA is used as the base.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com