Cyclobutyl-1-enamine compound, preparation method thereof and application of cyclobutyl-1-enamine compound in medicine
A compound and enamine technology, applied in the field of organic chemical synthesis, can solve the problems of blank research status, high price, unfriendly environment, etc., and achieve the effect of simple post-processing, short reaction path, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: the synthesis of TMS-EBX iodide 4
[0058]
[0059] The specific synthesis steps of TMS-EBX iodide 4 are as follows:
[0060] 1) Dissolve o-iodobenzoic acid 5 (7.44g, 30mmol) and sodium periodate (6.74g, 31.5mmol) in 30% (v:v) aqueous glacial acetic acid (50mL) and reflux for 4.0h until the reaction is complete. Add ice water (30mL) to the reaction system, and cool to room temperature under the condition of avoiding light, filter out the white solid, wash with ice water (60mL) and ice acetone (60mL), and dry at room temperature under avoiding light to obtain 1- Hydroxy-1,2-benzotriazol-3-one compound 6 (7.13 g, 27 mmol), yield 90%.
[0061] 2) Under nitrogen protection, put 1-hydroxy-1,2-benzotriazol-3-one 6 (5.28g, 20mmol) in a round bottom flask, add dichloromethane (30mL), and slowly Add trimethylsilyl trifluoromethanesulfonate (5.44mL, 30mmol), rise to room temperature and stir for 0.5h; then add bis(trimethylsilyl)acetylene (4.99mL, 22mmol), stir...
Embodiment 2
[0062] Embodiment 2: the synthesis of alkyne amide raw material 1a-1i
[0063] In particular, the abbreviation in the compound structure: Ts represents p-toluenesulfonyl, and Mbs represents p-methoxybenzenesulfonyl.
[0064]
[0065] Taking the specific synthesis steps of alkyne amide 1a as an example, add sulfonamide 3a (123.7mg, 0.50mmol) and cesium carbonate (211.8mg, 0.65mmol) to a 25mL round-bottomed flask in sequence, and seal the mouth of the bottle with a well-sealed flip-top rubber stopper And carry out nitrogen protection, then add dried N,N-dimethylformamide (1.0mL), stir at room temperature for 0.5h; dissolve TMS-EBX iodide 4 (258.2mg, 0.75mmol) in dichloromethane (2.5 mL), slowly added to the reaction system at 0°C in the dark, raised to room temperature and stirred for 0.5h; the reaction progress was monitored by thin-layer chromatography, and after the reaction was completed, it was filtered through silica gel, and the solvent was removed under reduced pressu...
Embodiment 3
[0085] Embodiment 3: the synthesis of compound 2a-2i
[0086]
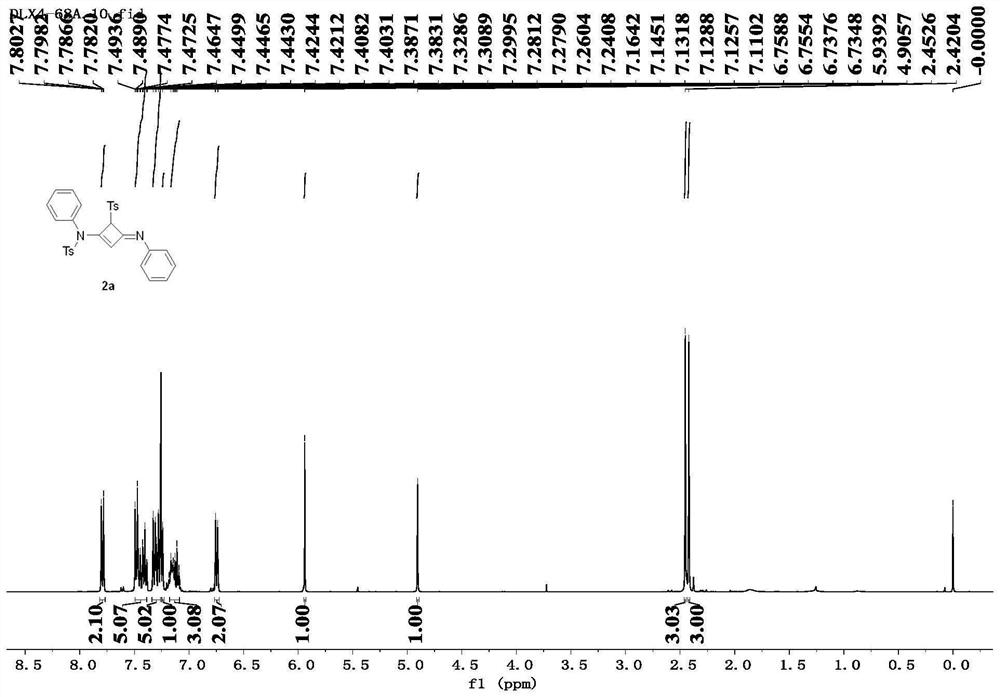
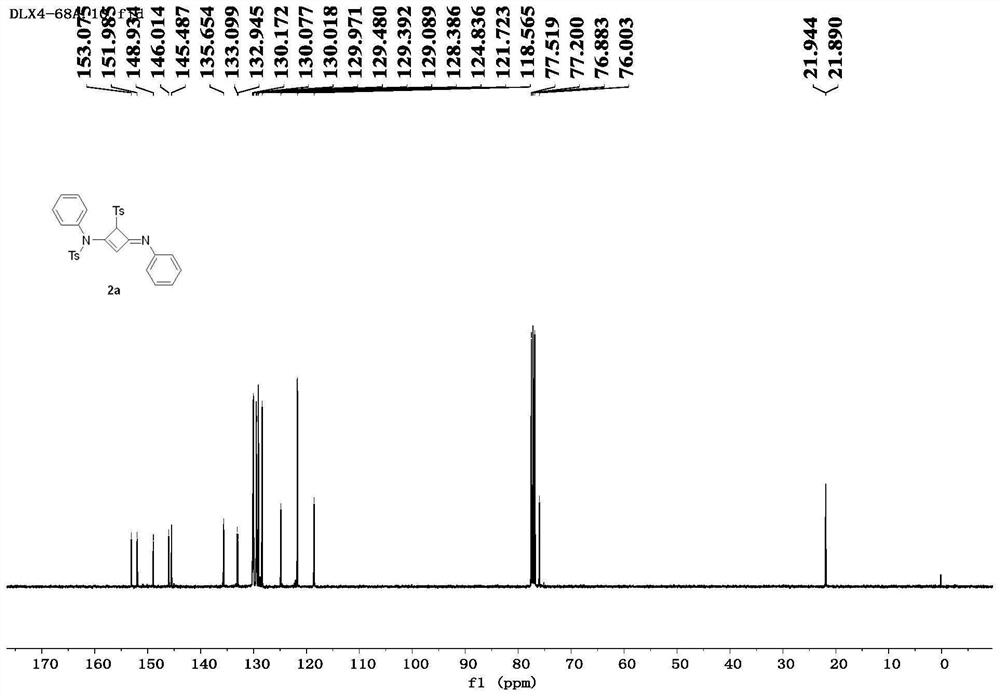
[0087] Taking the specific synthesis steps of compound 2a as an example, under the protection of nitrogen, add alkyne amide 1a (81.4mg, 0.3mmol) into a dry reaction flask, and heat the reaction system until it is in a molten state (100°C). Maintaining its molten state, the progress of the reaction was monitored by thin-layer chromatography, and the reaction was completed after 2.0 h. The obtained crude product was separated by column chromatography using petroleum ether / ethyl acetate (3.5:1) as eluent to obtain the white solid product 2a (70.8 mg, 0.131 mmol), with a yield of 87%.
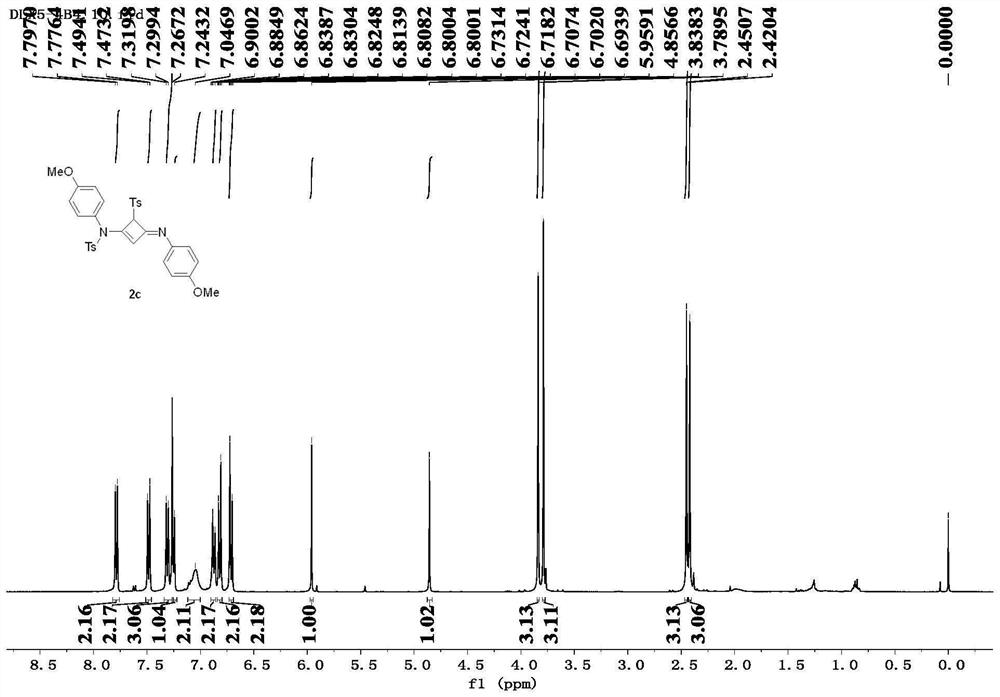
[0088] The alkyne amide intermediates 1b-1i prepared in Example 2 were prepared according to the above-mentioned method in this example to prepare compound 2, corresponding to obtain compound 2b-2i (Ts represents p-toluenesulfonyl, Mbs represents p-methoxybenzene sulfonyl).
[0089]
[0090] Compound 2a: white solid, 87% yield,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com