Fenbufen pharmaceutical co-crystal and preparation method thereof
A technology of drug and drug activity, which is applied in the field of fenbufen drug co-crystal and its preparation, can solve the problems of decreased solubility and achieve the effects of low cost, improved solubility and bioavailability, and improved oral absorption rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
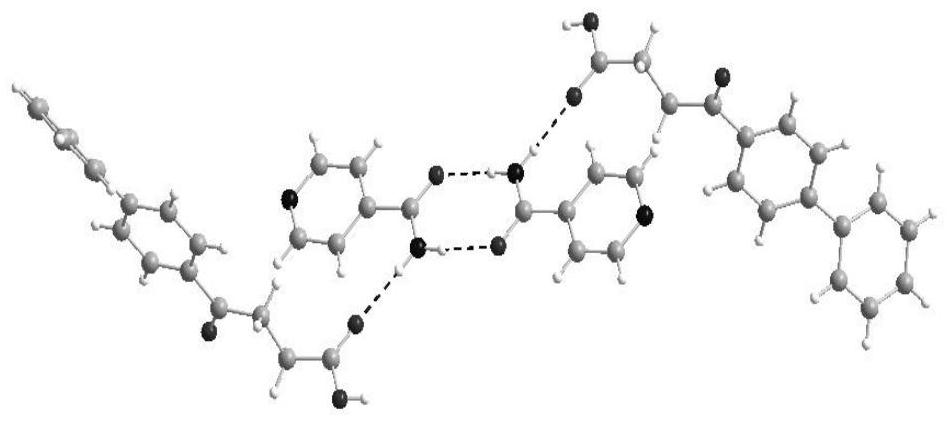
[0034] Weigh 254.2 mg of fenbufen and 122.2 mg of isonicotinamide, dissolve them in 10 ml of a mixed solvent of acetone and cyclohexane with a volume ratio of 1:1, ultrasonicate for 50 min, cool slowly, and filter the resulting suspension to obtain The clarified solution was placed in a constant temperature incubator at 35° C. for constant temperature cultivation. After 7 days, the fenbufen-isonicotinamide co-crystal was obtained. Using SXRD to characterize and analyze the crystal, the obtained fenbufen-isonicotinamide crystal structure is as follows: figure 1 As shown, its crystal structure belongs to the triclinic crystal system, and the space group is Shaft length: Angle: α / °=84.335(4), β / °=86.495(4), γ / °=80.888(4), Z=2.
Embodiment 2
[0036] Weigh 127.1mg of fenbufen and 122.2mg of isonicotinamide, dissolve in 12ml of acetonitrile and chloroform mixed solvent with a volume ratio of 2:1, sonicate for 80min, cool slowly, and filter the resulting suspension to obtain a clear solution Placed in a constant temperature incubator at 30°C for constant temperature cultivation, after 10 days, the fenbufen-isonicotinamide co-crystal was obtained. The crystals were characterized by differential scanning calorimetry, and the fenbufen-isonicotinamide cocrystal had an absorption peak at 143.31°C, and the DSC figure was as follows figure 2 shown.
Embodiment 3
[0038] Weigh 254.2mg of fenbufen and 61.1mg of isonicotinamide, dissolve in 15ml of a mixed solvent of ethanol and acetone with a volume ratio of 4:1, sonicate for 120min, cool slowly, and filter the resulting suspension to obtain a clear solution Placed in a constant temperature incubator at 25°C for constant temperature cultivation, after 15 days, the fenbufen-isonicotinamide co-crystal was obtained. Using PXRD to characterize the obtained crystals, the fenbufen-isonicotinamide cocrystals are at 2θ=5.8°, 8.9°, 12.0°, 17.9°, 19.6°, 20.9°, 21.6°, 23.9°, 25.8°, 27.1° , there are characteristic peaks at 30.1°, 36.3°, and 46.0°, and the characteristic curves are as follows image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com