Application of malic acid, vortioxetine hydrobromide oral instant film agent and preparation method of vortioxetine hydrobromide oral instant film agent
A technology of oral instant film and vortioxetine, which is applied in the direction of non-active ingredient medical preparations, active ingredient-containing medical preparations, pharmaceutical formulas, etc., which can solve the problem of affecting drug compliance and increasing nephrotoxicity Risk, product foaming and other issues, to achieve the effect of increasing crystal transparency, reducing PVA degradation, and enhancing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
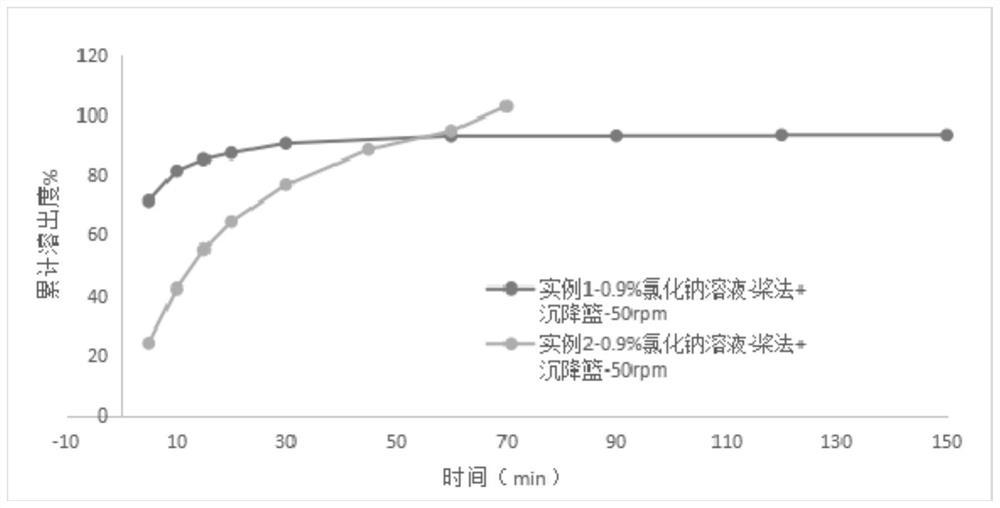
Image
Examples
Embodiment 1
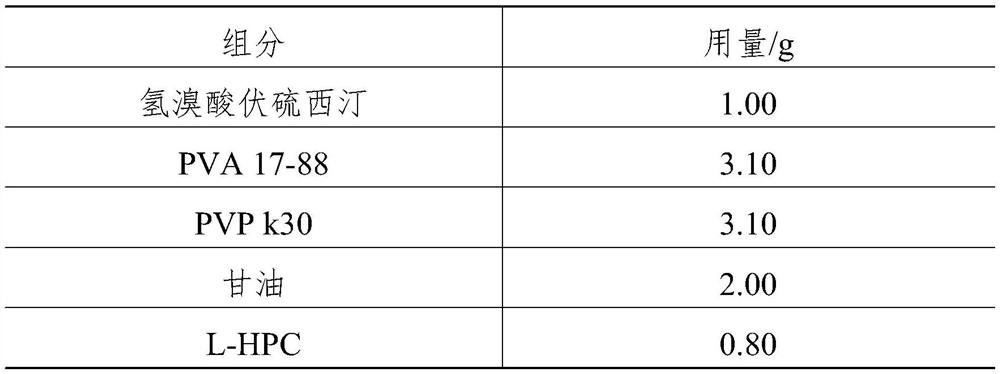
[0043] Vortioxetine hydrobromide orally dissolving film, it is prepared by the component of table 1 corresponding weight:
[0044] Table 1
[0045] components Dosage / g Vortioxetine hydrobromide 4.00 PVA 17-88 6.98 PVP k30 6.98 Soy lecithin 4.00 Sorbitol 4.00 glycerin 8.00 L-HPC 3.20 malic acid 2.40 stevioside 0.40 sodium bisulfite 0.04
[0046] The preparation method is specifically:
[0047] S1 nano-grinding vortioxetine hydrobromide to reduce particle size;
[0048] S2 Weigh PVP K30, L-HPC, vortioxetine hydrobromide, malic acid, sorbitol, soybean lecithin, stevioside, sodium bisulfite and glycerin of the corresponding weight in Table 1, add to purified water and stir to dissolve / Disperse to obtain a suspension;
[0049] S3 Weigh the corresponding weight of PVA17-88 in Table 1 and add it into purified water, stir and dissolve in a water bath at 70°C, lower the temperature to 50°C, and stir with th...
Embodiment 2
[0053] Vortioxetine hydrobromide orally dissolving film, it is prepared by the component of table 2 corresponding weight:
[0054] Table 2
[0055] components Dosage / g Vortioxetine hydrobromide 4.00 PVA 17-88 6.98 PVP k30 6.98 Soy lecithin 4.00 Sorbitol 4.00 glycerin 8.00 L-HPC 3.20 malic acid 2.40 stevioside 0.40 sodium bisulfite 0.04
[0056] The preparation method is specifically:
[0057] S1 Weigh PVP K30, L-HPC, vortioxetine hydrobromide, malic acid, sorbitol, soybean lecithin, stevioside, sodium bisulfite and glycerin of the corresponding weight in Table 2, add to purified water and stir to dissolve / Disperse to obtain a suspension;
[0058] S2 Weigh the PVA17-88 of the corresponding weight in Table 2 and add it into purified water, stir and dissolve in a water bath at 70°C, then lower the temperature to 50°C, and stir with the suspension for 2 hours to obtain a glue;
[0059] S3 Ultrasonicall...
Embodiment 3
[0062] Vortioxetine hydrobromide orally dissolving film, it is prepared by the component of table 3 corresponding weight:
[0063] table 3
[0064]
[0065] The preparation method is specifically:
[0066] S1 nano-grinding vortioxetine hydrobromide to reduce particle size;
[0067] S2 Weigh the PVP K30, L-HPC, vortioxetine hydrobromide and glycerin of the corresponding weight in Table 3, add to purified water and stir to dissolve / disperse to obtain a suspension;
[0068] S3 Weigh the corresponding weight of PVA17-88 in Table 3 and add it into purified water, stir and dissolve in a water bath at 70°C, then lower the temperature to 50°C and stir with the suspension for 2 hours to obtain a glue
[0069] S4 Ultrasonically remove the air bubbles from the obtained glue, control the temperature not to exceed 50°C, and after the end of the ultrasound, lower it to room temperature; use a coating machine to evenly coat the glue on the PET film to prepare a drug-loaded orally dissol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com