Pharmaceutical composition and application thereof in preparation of anti-osteoporosis drugs
A composition and drug technology, applied in the field of diabetes treatment, can solve the problems of inability to block key pathological links of diabetic osteoporosis, mandibular necrosis, complicated pathogenesis of diabetic osteoporosis, etc., and achieve reduction of tartrate-resistant acidic phosphate Enzyme activity, increase alkaline phosphatase activity, good effect of hypoglycemic drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A pharmaceutical composition, in terms of the amount of substances, comprising: 19.69 parts of mangiferin, 6.01 parts of tetrahydroepiperberine, 33.26 parts of cortex, 12.94 parts of magnolialine, 2.92 parts of 13-hydroxyl oxidized berberine, 0.22 1 part palmatine and 24.96 parts berberine.
[0048] According to the amounts of the above substances, the raw materials are mixed to obtain the pharmaceutical composition of this embodiment.
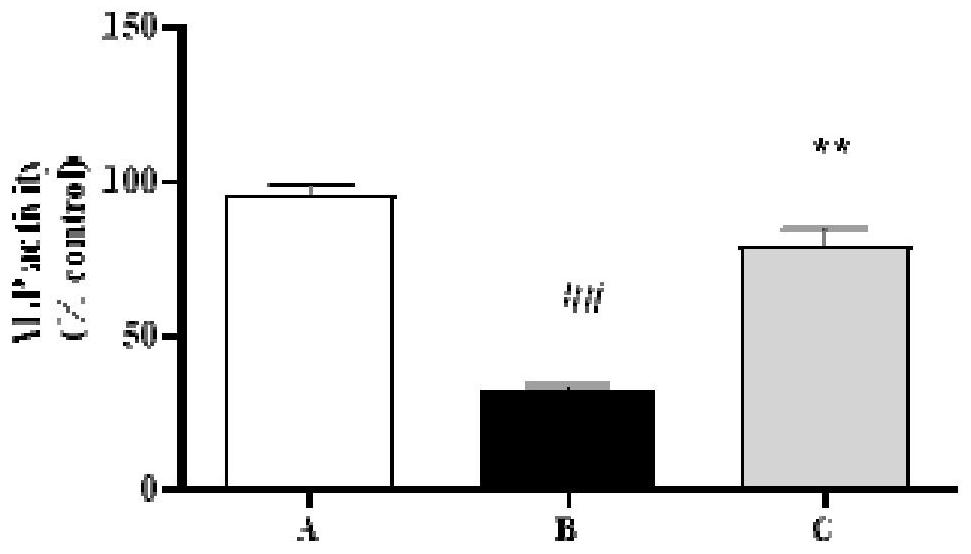
[0049] In order to study the anti-diabetic osteoporosis function of the pharmaceutical composition of this embodiment, the zebrafish of the AB line was taken as the test object, and the juvenile fish experiment and the adult fish experiment were respectively carried out; wherein, the juvenile fish experiment was used to observe the effect of the pharmaceutical composition on the skull. The effect of bone mass and optical density, and fish experiments are used to observe the effects of pharmaceutical compositions on promoting bone format...
Embodiment 2
[0073] A pharmaceutical composition, in parts by weight, comprising: 7.64 parts of mangiferin, 7.39 parts of tetrahydroepiperberine, 35.06 parts of cortex, 14.64 parts of magnolanine, 4.26 parts of 13-hydroxyl oxidized berberine, 0.29 parts palmatine and 30.71 parts berberine.
[0074] According to the amounts of the above substances, the raw materials are mixed to obtain the pharmaceutical composition of this embodiment.
Embodiment 3
[0076] A pharmaceutical composition, in parts by weight, comprising: 10.40 parts of mangiferin, 7.06 parts of tetrahydroepiperberine, 35.31 parts of cortex, 13.86 parts of magnolialine, 3.63 parts of 13-hydroxyloxyberberine, 4.26 parts palmatine and 29.47 parts berberine.
[0077] According to the parts of the above substances, the raw materials are mixed to obtain the pharmaceutical composition of this embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com