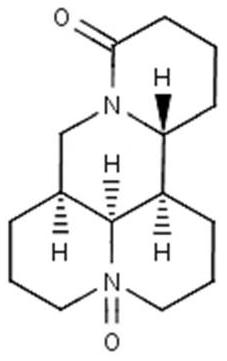
Kushenin tablet and preparation method thereof
A technology of matrine and ginseng tablets, which is applied in the direction of medical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve incomplete release, easy to produce sticky punches, and unstable drug release and other problems, to achieve the effects of stable drug release, qualified content uniformity, and suitable for commercial scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 matrine tablet
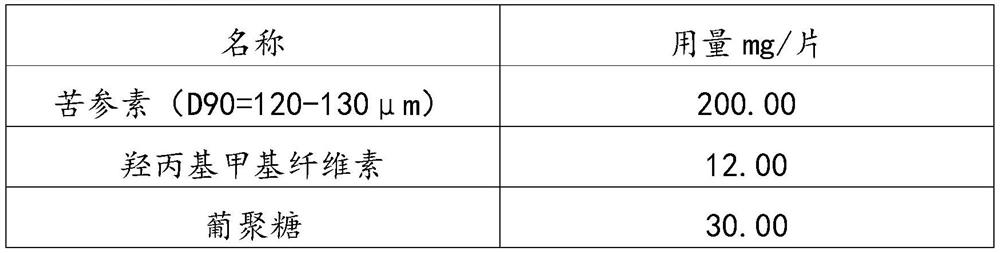
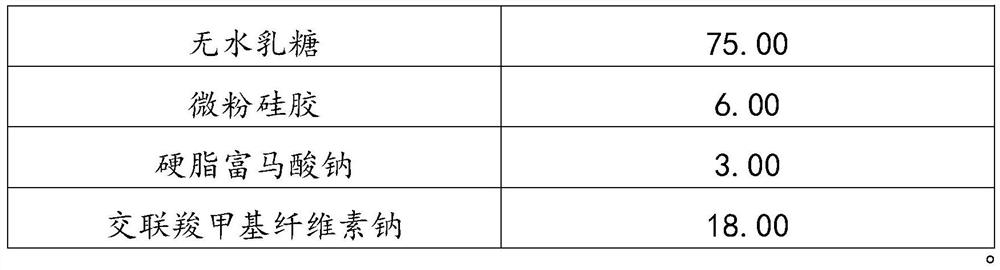
[0032] The prescription composition of 1000 described matrine tablets
[0033] name Dosage (g) Matrine (D90=120-130μm) 200.00 Hydroxypropylmethylcellulose 12.00 Dextran 30.00 anhydrous lactose 75.00 Micropowder silica gel 6.00 Sodium stearyl fumarate 3.00 Croscarmellose Sodium 18.00
[0034] Preparation:
[0035] 1) Grinding matrine to a particle size D90 of 120-130 μm to obtain matrine powder;
[0036] 2) Separately pulverize hydroxypropyl methylcellulose, dextran, anhydrous lactose, croscarmellose sodium, micropowder silica gel, and sodium stearyl fumarate, pass through a 100-150 mesh sieve, and set aside;
[0037] 3) Add the sieved micro-powder silica gel to the matrine powder obtained in step 1), and stir at a speed of 300-500r / min for 10-15min to obtain a matrine dispersion;
[0038] 4) Add sieved hydroxypropyl methylcellulose and dextran to the matrine disper...
Embodiment 2
[0040] The preparation of embodiment 2 matrine tablets
[0041] The prescription composition of 1000 described matrine tablets
[0042]
[0043]
[0044] The preparation method is as in Example 1.
Embodiment 3
[0045] The preparation of embodiment 3 Kushensu Tablets The prescription composition of 1000 Kushensu Tablets
[0046] name Dosage (g) Matrine (D90=110-120μm) 200.00 Hydroxyethyl cellulose 18.00 Dextran 36.00 Calcium Dihydrogen Phosphate 100.00 Micropowder silica gel 16.00 Magnesium stearate 4.00 Crospovidone 30.00
[0047] The preparation method is as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com