Nano vaccine as well as preparation method and application thereof
A nano-vaccine and nano-particle technology, applied in the field of biomedicine, can solve problems such as poor solubility and application limitations, and achieve the effects of low toxicity and side effects, large drug loading, and significant lethality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
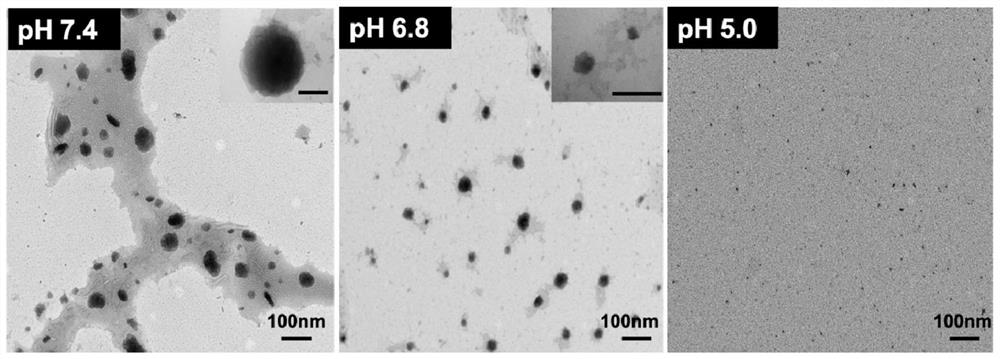
[0035] Example 1: Preparation of nano-vaccine, verification of pH-responsiveness of nano-vaccine
[0036] A nano-vaccine in response to acid stimulation, which is prepared through the following steps:
[0037] (1) Pour 5 mL of chloroform into a clean round bottom flask with a graduated cylinder, then add PEG-hyd-PCL (25 mg) and PEI-PCL (12.5 mg) into the round bottom flask, add magnets and stir on a magnetic stirrer until dissolved; after fully dissolved, evaporate in a rotary evaporator at 60°C. After the chloroform was completely evaporated to form a thin film, 3ml of deionized water at a temperature of 65°C was slowly dropped into it while stirring, and further stirred at room temperature for 4h, then dialyzed and washed to obtain unloaded hybrid micelles (HM).
[0038](2) Accurately weigh 0.25mg BLZ-945 and 0.5mg NLG-919 and place them in a centrifuge tube, add 0.05mL DMSO, vortex to fully dissolve, then add 1mg of HM prepared in step (1) to the solution , mixed evenly, ...
Embodiment 2
[0041] Example 2: In vitro anti-tumor activity of nanomicelle vaccine complex (BN@HM-OVA) and its cellular immune response analysis
[0042] (1) Antigen uptake by myeloid-derived dendritic cells (BMDCs)
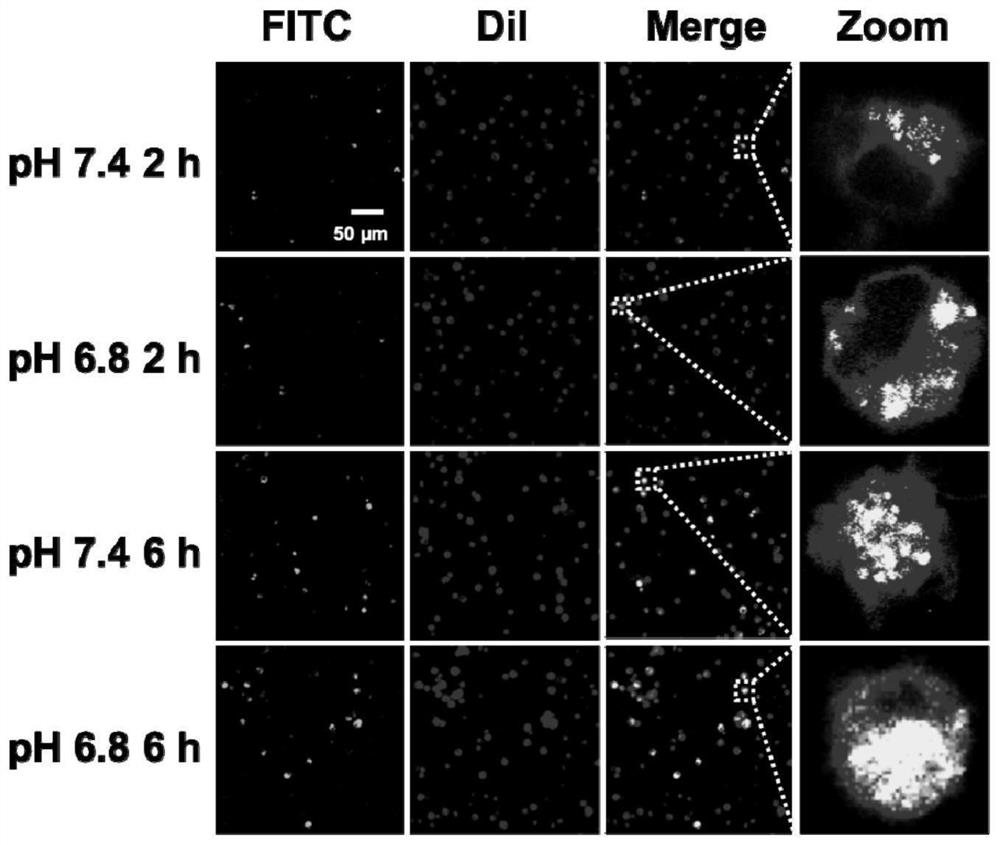
[0043] Whether DCs cells can successfully uptake antigens is the key to promoting their maturation. Mouse bone marrow cells were induced to BMDCs on day 5 in vitro, and BMDCs were stained with cell membrane dye Dil. According to 1×10 6 / mL BMDCs The stained BMDCs were co-incubated with 10 μL BN@HM-OVA-FITC (OVA-FITC concentration 10 μg / mL) in different pH (pH 7.4, pH 6.8) media for different periods of time (2h, 6h). The uptake of BN@HM-OVA-FITC by BMDCs was detected by laser scanning confocal microscopy.
[0044] From image 3 The respective confocal images showed that BN@HM-OVA-FITC could be efficiently taken up by BMDCs in a time-dependent manner after 2 h and 6 h incubation at pH 7.4 and 6.8. Furthermore, regardless of the incubation time, BMDCs at pH 6.8 internalize...
Embodiment 3
[0051] Embodiment 3: nano vaccine tumor live treatment study
[0052] (1) Anti-tumor effect of nano-vaccine complexes in prophylactic administration mode on tumor-bearing mice
[0053] To evaluate the effectiveness of the nanovaccine in tumor prevention, six-week-old C57BL / 6 mice were immunized using a homologous prime-boost regimen. Mice were divided into six groups (n=6 per group), one group was injected with Saline, one group was injected with Free OVA, and four groups were injected with HM-OVA for immunization (OVA: 5 nmol per mouse), and at designated time points Injected subcutaneously into both footpads of mice. Three weeks after the last immunization, each mouse was subcutaneously inoculated with 2×10 5 E. G7-OVA cells. Starting on the 6th day after tumor inoculation, every 4 days, 3 groups of mice immunized with HM-OVA were injected with Free NLG-919, FreeBLZ-945 and Free NLG-919+Free BLZ-945, total Dosing 5 times. Tumor growth was monitored every two days using ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com