Content determination method of deoxycholic acid injection
A technology of deoxycholic acid and a determination method, which is applied in the directions of measuring devices, instruments, scientific instruments, etc., can solve the problems such as few reports on the content determination method of deoxycholic acid injection, and achieve the advantages of good quality of medicine and high accuracy. , good quantitative ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] The drug content assay method of the deoxycholic acid injection of the present invention of embodiment 1
[0090] 1. Instruments and analysis conditions
[0091] High performance liquid chromatography (Waters e2695), electrospray detector (model Corona Veo), chromatographic column is Agilent Pursuit C18, 3 μ m, 4.6 * 150mm, mobile phase A is 0.1% formic acid aqueous solution, and mobile phase B is 0.1% formic acid acetonitrile Solution, the elution condition is A phase: B phase = 35:65 isocratic elution, column temperature 35°C, flow rate 1.0ml / min, injection volume 25μl, electrospray detector parameters: T = 35°C, acquisition frequency 10HZ, filter=5.0s, injector temperature 5°C, running time 9 minutes.
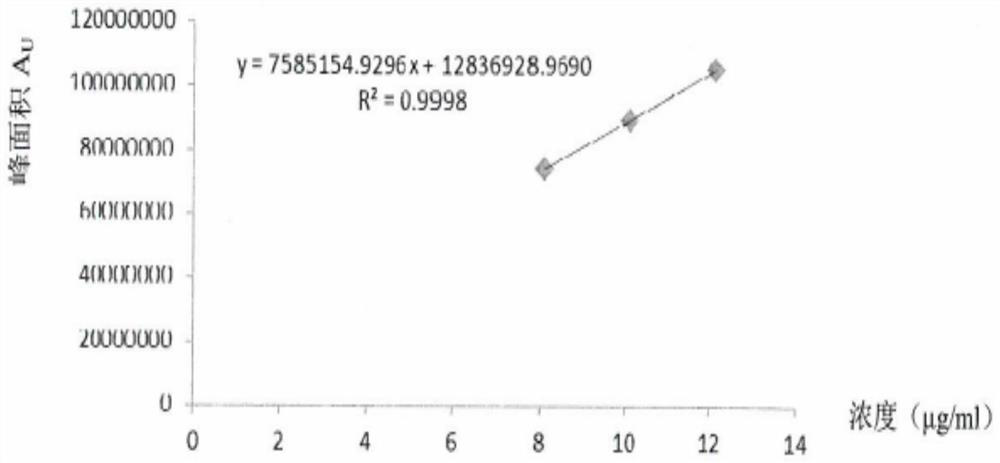
[0092] 2. Preparation of solvent, standard solution and test solution
[0093] Solvent: the solvents described in the present invention are all 80% methanol solution, measure 800ml methanol and 200ml water respectively with a graduated cylinder, mix them with ultras...
Embodiment 2
[0116] Embodiment 2 The system suitability investigation test of assay method of the present invention
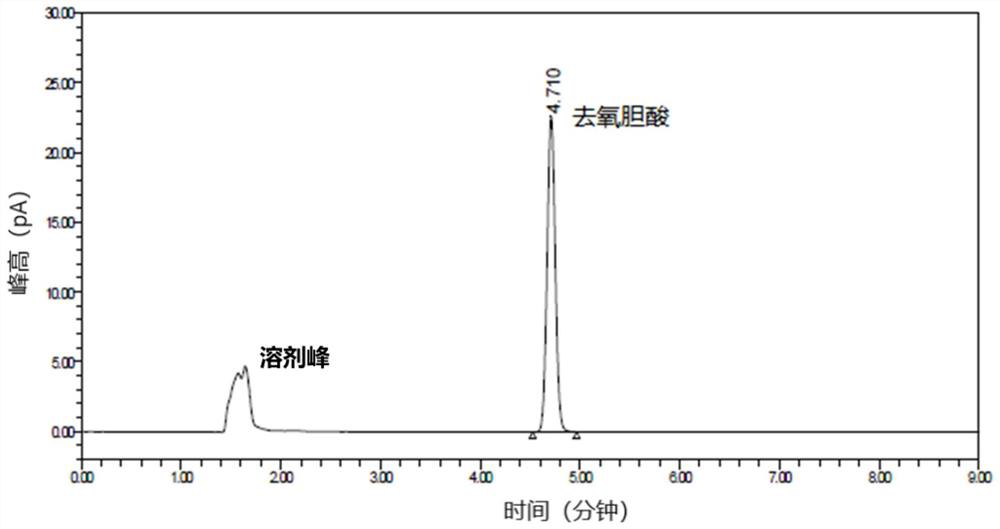
[0117] The purpose of this experiment is to confirm whether the content test method in Example 1 meets the requirements of system applicability. The acceptance criteria for the system applicability investigation experiment is: the RSD of the deoxycholic acid peak area of 6-pin standard solution 2 should be ≤ 3.0%, the RSD of the retention time should be ≤1.0%, and the deoxycholic acid peak tailing factor of the first injection of standard solution 2 should be ≤1.8.
[0118] 1. Instrument and analysis conditions: with embodiment 1.
[0119] 2. Solution preparation: prepare the standard solution and the test solution with reference to the method of Example 1.
[0120] 3. Experimental process:
[0121] Take 25 μl of the solvent, inject it into the high-performance liquid chromatograph, and record the chromatogram. The solvent should have no interference. Take 25 μl of sta...
Embodiment 3
[0125] The specificity investigation of embodiment 3 assay method of the present invention
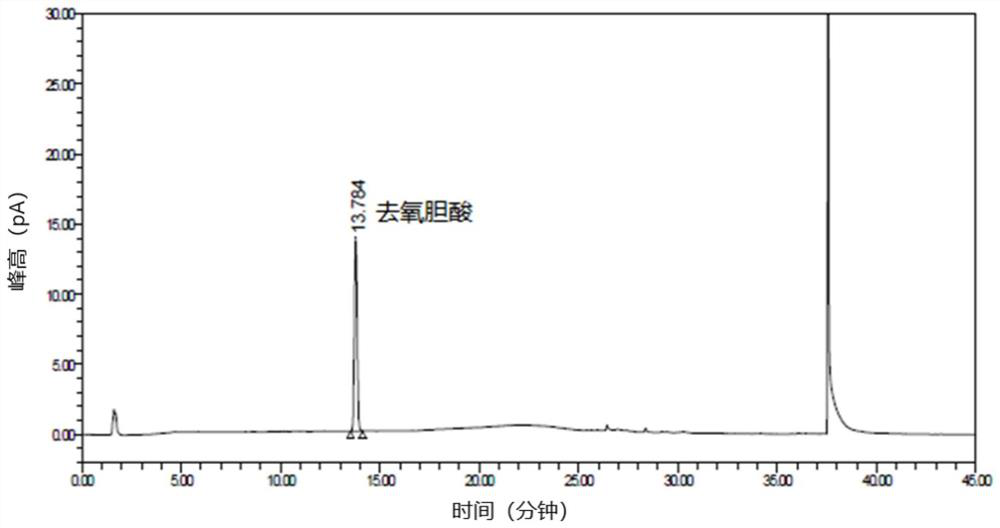
[0126] The purpose of this experiment is to verify that the content determination method of the present invention can completely separate deoxycholic acid peaks from all known impurities and potential impurities, and that the content test is not interfered by impurity peaks, so as to prove that the content test method of the present invention has good specificity.
[0127] 1. Instrument and analysis condition are with embodiment 1.
[0128] 2. Solution preparation
[0129] Excipient blank solution: accurately measure 5.0ml of placebo, put it in a 50ml measuring bottle, add solvent to dilute to the mark, shake well, precisely measure 1.0ml of the above solution, put it in a 100ml measuring bottle, add solvent to dilute to the mark, shake well, Instantly.
[0130] Undamaged sample: Accurately measure 5.0ml of deoxycholic acid injection, put it in a 50ml measuring bottle, add solvent to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com