Preparation method of fluorinated acyl fluoride
A technology of acyl fluoride and hydrogen fluoride, which is applied in the field of preparation of acyl fluoride, can solve the problems of cumbersome separation steps, unstable yield, and high requirements for production equipment, and achieve a wide range of raw material sources, reduced production costs, and a high degree of automation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
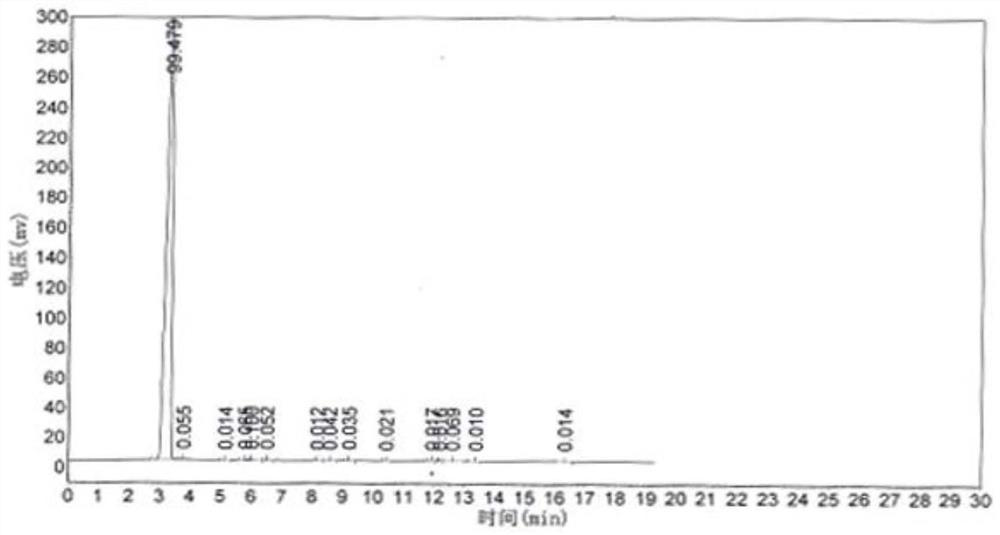
Embodiment 1
[0048] The concrete steps of the preparation method of the difluoroacetyl fluoride of the present embodiment are:
[0049] First, heat the premixer to 110°C to 115°C with heat conduction oil, and heat the reactor filled with the loaded 3-difluoromethyl-1H-methylpyrazole-4-carboxylic acid chromium catalyst to 180°C to 190°C ℃, use nitrogen to purge from the raw material inlet or hydrogen fluoride inlet to ensure that there is no moisture in the entire reaction device.
[0050] Then start the premixer to stir, and start to feed hydrogen fluoride gas into the premixer (flow rate: 5m³ / h). After hydrogen fluoride escapes from the product outlet at the top of the reactor, feed trichlorethylene and dry oxygen into the premixer at the same time (Trichlorethylene flow rate: 1.5m³ / h, oxygen flow rate: 1.8m³ / h), the reactor back pressure is 0.1MPa and the reaction is carried out at 180℃~190℃, and the mixed gas of difluoroacetyl fluoride, hydrogen chloride and hydrogen fluoride can be obt...
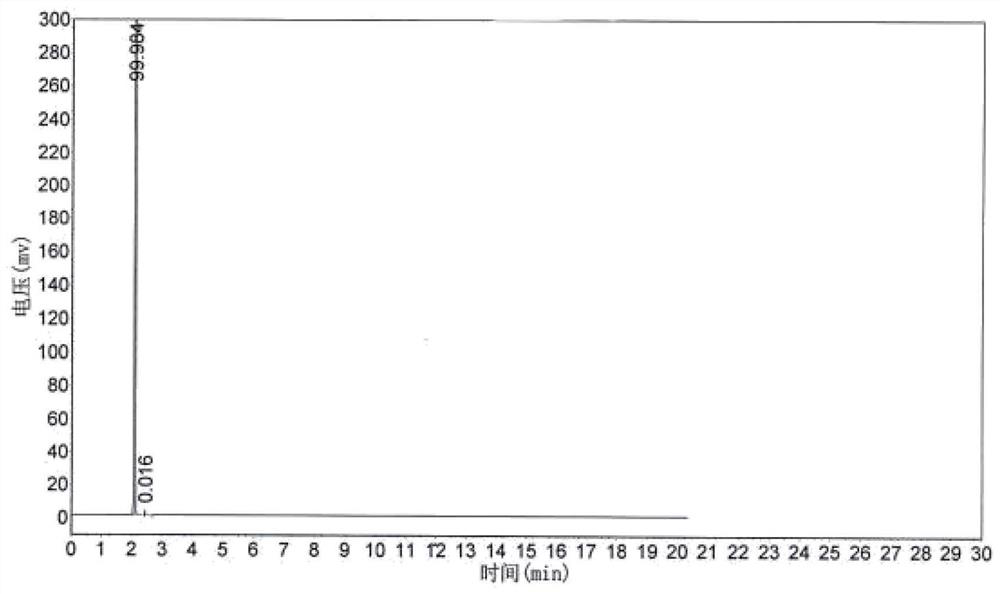
Embodiment 2
[0065] The concrete steps of the preparation method of the nonafluorovaleryl fluoride of the present embodiment are:
[0066] Firstly, heat the premixer to 160°C-165°C with heat conduction oil, heat the reactor filled with the loaded complex catalyst to 170°C-175°C, and use nitrogen gas from the raw material inlet or hydrogen fluoride inlet. Purge to ensure that the entire reaction apparatus is free of moisture.
[0067] Then start the premixer to stir, start to feed hydrogen fluoride gas into the premixer (flow rate: 5m³ / h), and after the hydrogen fluoride escapes from the product outlet at the top of the reactor, the 4,5,5,5-decachloropent-1-ene and dry oxygen are simultaneously fed into the premixer (1,1,2,3,3,4,4,5,5,5-decachloropent-1 -Oxygen flow rate: 1.3m³ / h, oxygen flow rate: 1.8m³ / h), reactor back pressure 0.2MPa, react at 170℃~175℃, can stably obtain mixed gas of nonafluorovaleryl fluoride, hydrogen chloride and hydrogen fluoride. The reaction formula is as follow...
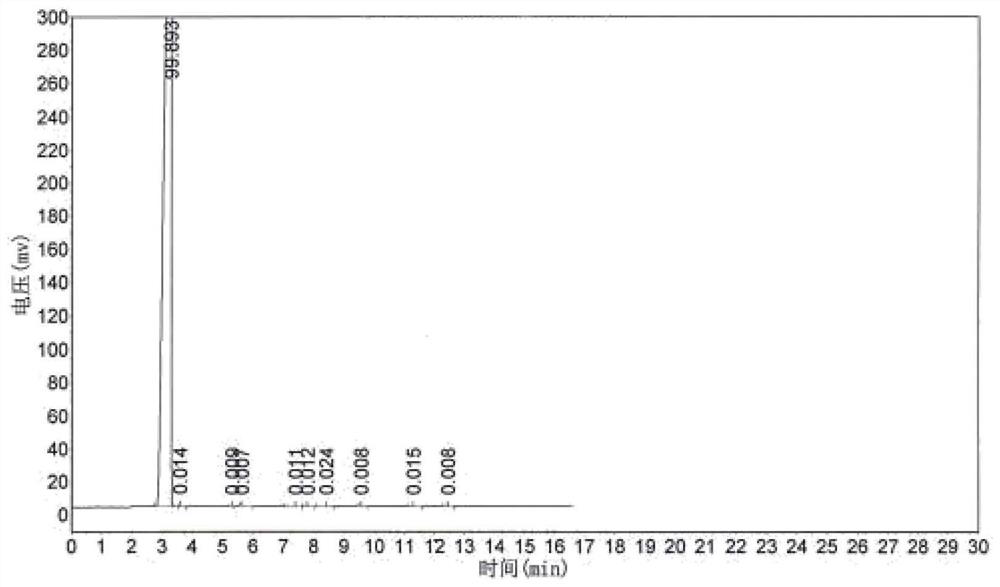
Embodiment 3
[0074] The concrete steps of the preparation method of the trifluoroacetyl fluoride of the present embodiment are:
[0075] Firstly, heat the premixer to 120°C~125°C with heat transfer oil, heat the reactor filled with the loaded complex catalyst to 150°C~155°C, and use nitrogen gas from the raw material inlet or hydrogen fluoride inlet. Purge to ensure that the entire reaction apparatus is free of moisture.
[0076] Then start the premixer to stir, start to feed hydrogen fluoride gas into the premixer (flow rate: 5m3 / h), until hydrogen fluoride escapes from the product outlet at the top of the reactor, then feed tetrachlorethylene and dry oxygen into the premixer at the same time ( Tetrachlorethylene: 1.7m³ / h, oxygen flow rate 1.7m³ / h), reactor back pressure 0.1MPa, reaction at 150℃~155℃, can stably obtain the mixed gas of trifluoroacetyl fluoride, hydrogen chloride and hydrogen fluoride. The reaction formula is as follows:
[0077] .
[0078] Since the detection of trif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com