Inhalation ueclidinium bromide solution preparation and preparation method thereof
A technology of umeclidinium bromide and solution, which is applied in the field of pharmaceutical preparations, can solve the problems of irritation, inability to achieve drug delivery, tracheal spasm adverse reactions, etc., and achieve the effects of reducing irritation, preparation safety, and increasing compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
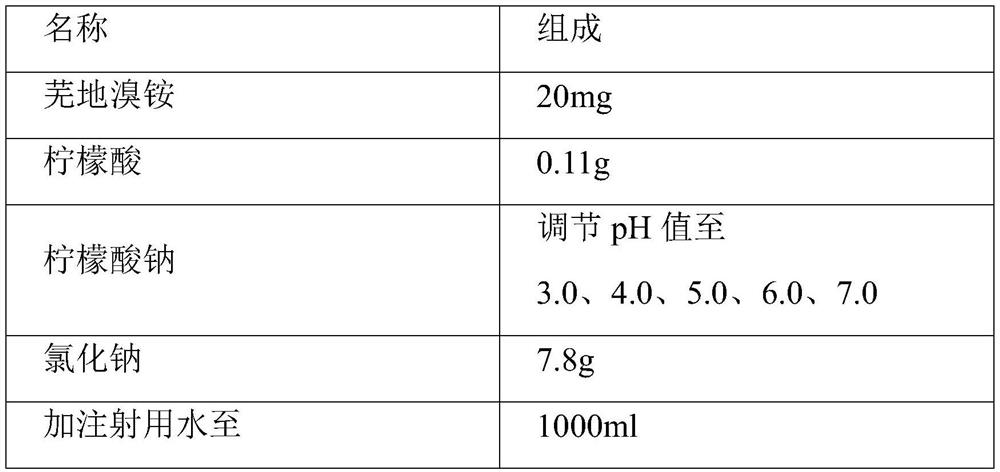
[0042] prescription composition
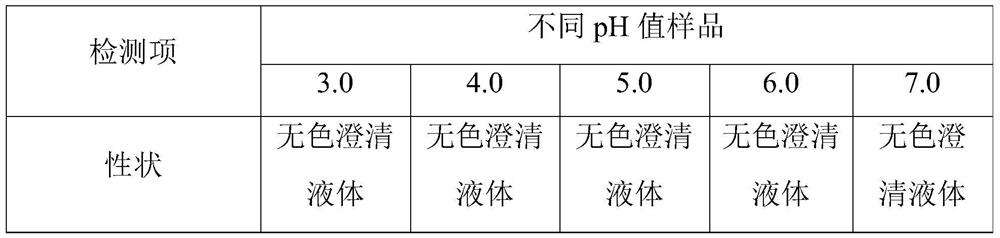
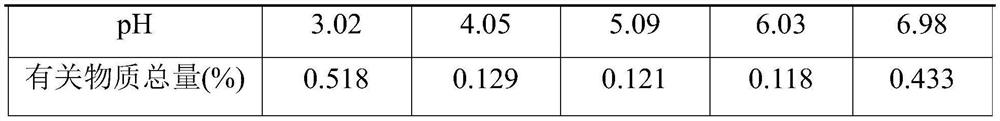
[0043] name composition Umeclidinium Bromide 20mg citric acid 0.11g Sodium citrate Adjust pH to 4.5 Sodium chloride 7.8g Add water for injection to 1000ml
[0044] Preparation:
[0045] Measure 800ml (80% of prescription quantity) of water for injection at room temperature, drop into prescription quantity sodium chloride, citric acid successively, drop into umeclidinium bromide after stirring and dissolving, continue stirring to dissolve, add sodium citrate to adjust pH value to 4.5 , after stirring evenly, make up to the full amount with a solvent, filter with a 0.22 μm filter membrane or filter element, fill into a 1ml ampoule bottle, seal it, and get ready.
Embodiment 2
[0047] prescription composition
[0048] name composition Umeclidinium Bromide 40mg Dilute hydrochloric acid Appropriate amount sodium bicarbonate Adjust pH to 6.0 Sodium chloride 8.7g Add water for injection to 1000ml
[0049] Preparation:
[0050] Measure 700ml of water for injection at 40°C (70% of the prescribed amount), put into the prescribed amount of sodium chloride, stir and dissolve, add dropwise dilute hydrochloric acid until the pH value reaches 3.0, stir evenly, put in umeclidinium bromide and stir to dissolve, put in carbonic acid Adjust the pH value to 6.0 with sodium hydrogen, stir evenly, make up to the full amount with a solvent, filter with a 0.22 μm filter membrane or filter element, fill it into a 2ml ampoule bottle, and seal it.
Embodiment 3
[0052] prescription composition
[0053] name composition Umeclidinium Bromide 60mg citric acid 0.25g Sodium citrate Adjust pH to 5.0 glucose 48g Add water for injection to 1000ml
[0054] Preparation:
[0055] Measure the water for injection 800ml (80% of prescription quantity) of 60 ℃, drop into prescription quantity glucose and citric acid successively, stir and make dissolving, drop into umeclidinium bromide, after stirring makes dissolving, drop into sodium citrate and adjust pH value to 5.0, Stir evenly, make up to full amount with solvent, filter with 0.22μm filter membrane or filter element, fill into 2ml ampoules, seal, and get ready.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com