Diaryl heptane dimers, pharmaceutical composition thereof, and preparation method and application of two
A technology of diarylheptane and dimer, applied in the field of medicine, can solve the problem of no compound and the like, and achieve the effects of convenient operation, high yield and simple and easy preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
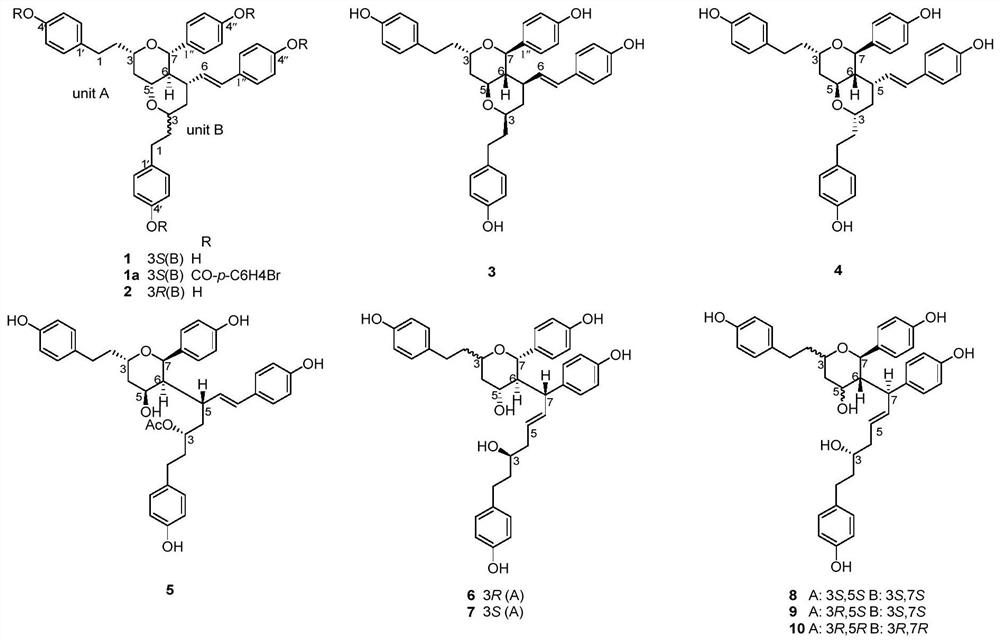
[0031] Preparation of Compound 1-10:
[0032] Dried seeds (20kg) of Cardamom grassa, crushed, extracted twice with 90% ethanol under reflux, each time for 2 hours, combined ethanol extracts, recovered ethanol under reduced pressure to obtain extract. The extract was dispersed in water and extracted with ethyl acetate, then concentrated to the ethyl acetate extract. Then the ethyl acetate extract (Fr.A, 1.5kg) was subjected to silica gel column chromatography, and methanol-chloroform (0:100, 2:98, 5:95, 10:90, 20:80 and 100:0, v / v) Eight fractions of Fr.A-1 to Fr.A-8 were obtained by gradient elution of the eluent. Fr.A-7 (50g) was obtained by MCI CHP 20P gel column chromatography (methanol-water, 30:70, 40:60, 50:50, 70:30, 100:0, v / v) to obtain Fr.A -7-1~Fr.A-7-6. Fr.A-7-2 (15g) was purified by silica gel column chromatography (MeOH-CHCl 3 , 10:90 and 20:80) obtained Fr.A-7-2a~Fr.A-7-2e. Fr.A-7-2c (5g) was purified by Sephadex LH-20 (chloroform-methanol, 50:50), silica g...
Embodiment 2
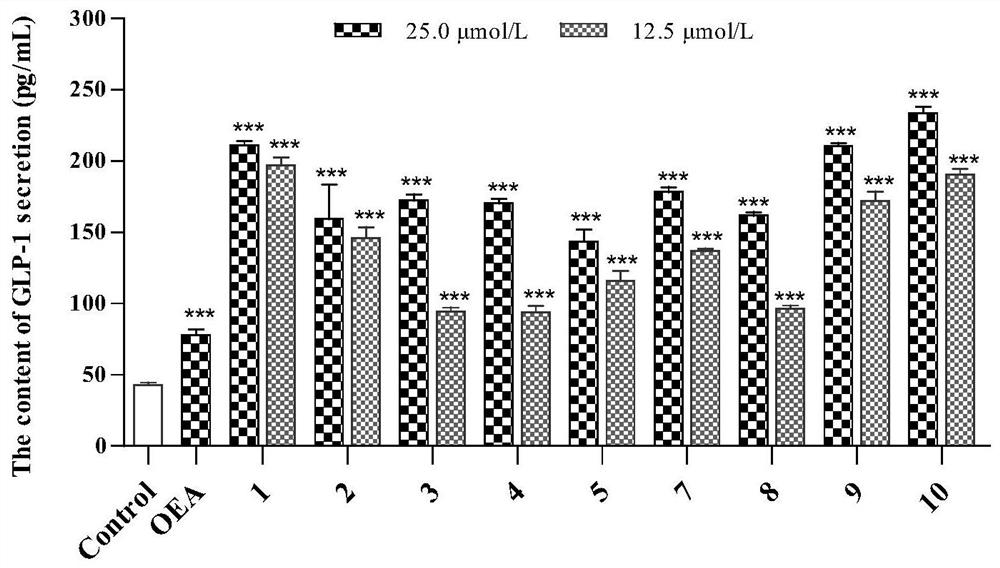
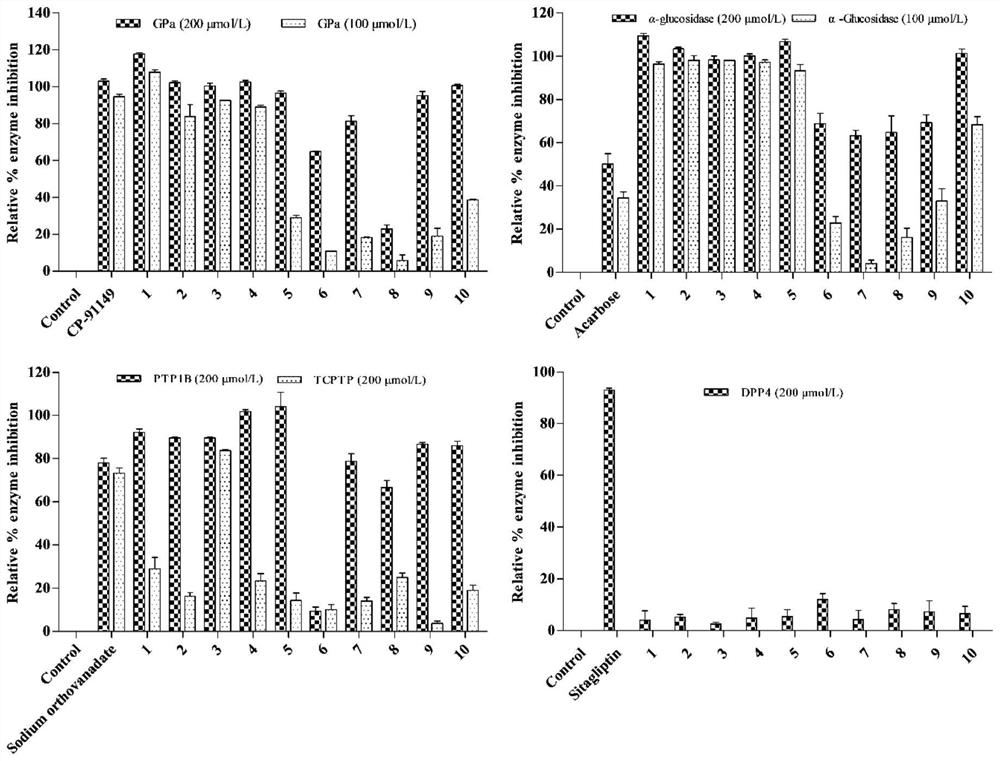
[0154] Compounds promote GLP-1 secretion and GPa, PTP1B, TCPTP and α-glucosidase inhibitory activity.
[0155] 1 Materials and methods
[0156] 1.1 Materials
[0157] STC-1 cells were purchased from ATCC, USA; α-glucosidase and GPa were purchased from Sigma Aldrich (St.Louis, MO, USA); phosphate buffer (≥99%, Meilun Biotech, Dalian); p-nitrobenzene Base-α-D-glucopyranose (≥99%, Yuanye Biotechnology, Shanghai); Acarbose (≥98%, Bayer Pharma, Beijing); PTP1B (protein tyrosine phosphatase) and TCPTP (T- Cell tyrosine phosphatase) was purchased from Sino Biological (Wayne, PA, USA); suramin sodium was purchased from ACROS (New Jersey USA); Hepes was purchased from Beijing Xiasi Biotechnology Co., Ltd. (Beijing); Lun Biotechnology Co., Ltd. (Dalian); α-D-glucose 1-phosphate disodium salt (St.Louis, MO, U.S.A.); ammonium molybdate was purchased from Shanghai Jiuding Chemical Co., Ltd. (Shanghai); malachite green was purchased from China Beijing Balingwei Technology Co., Ltd. (Beij...
preparation Embodiment
[0181] 1. Take any one or any combination of compounds 1-10, dissolve it with a small amount of DMSO, add water for injection as usual, fine filter, potting and sterilize to make an injection.
[0182] 2. Take any one of compounds 1-10 or any combination thereof, dissolve it in a small amount of DMSO, dissolve it in sterile water for injection, stir to dissolve, filter with a sterile suction filter funnel, and then filter aseptically, Packed in ampoules, freeze-dried at low temperature and sealed aseptically to obtain powder injection.
[0183] 3. Take any one of the compounds 1-10 or any combination thereof, and add the excipient at a ratio of 9:1 by weight to the excipient to make a powder.
[0184] 4. Take any one of compounds 1-10 or any combination thereof, add excipients at a weight ratio of 5:1 to excipients, granulate and compress into tablets.
[0185] 5. Take any one of compounds 1-10 or any combination thereof, and make oral liquid according to the conventional ora...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com