Adjuvant of novel coronavirus vaccine, application of adjuvant and novel coronavirus bivalent recombinant vaccine
A coronavirus and vaccine technology, applied in antiviral agents, viruses, vaccines, etc., can solve the problems of poor protection of the South African mutant strain B.1.351
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
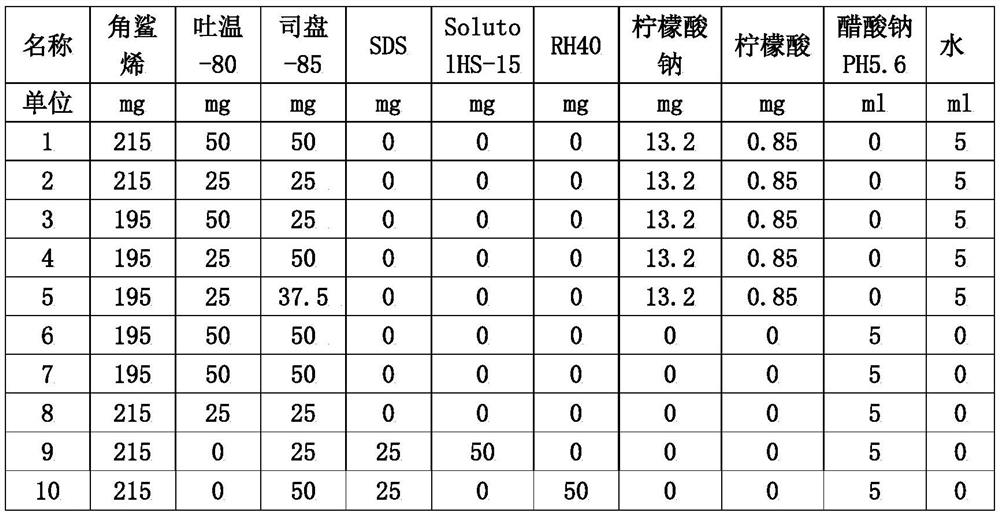
[0025] Embodiment 1, adjuvant configuration and optimization
[0026] 1. Adjuvant formula
[0027] Since the main components of the reference positive control MF59 adjuvant are squalene, Tween 80, and Span 85, and the buffer is a citric acid system. Sodium acetate buffer system is more stable, therefore, the adjuvant optimization of citrate buffer system and sodium acetate buffer system was carried out respectively, at the same time, SDS, Solutol HS-15 and RH40, which have similar protein protection functions as Tween 80, were added Research.
[0028] The configuration of the adjuvant The adjuvant test formula is as follows:
[0029]
[0030] Weigh squalene, Tween-80 and Span-85, or / and SDS, stir and mix evenly, as the oil phase. Add to the aqueous phase of the acetic acid-sodium acetate solution. Mix the oil phase and the water phase, stir them separately with a disperser, 1000-10000 rpm, stir for 5-20 minutes to form colostrum, and then process it through a micro-flui...
Embodiment 2
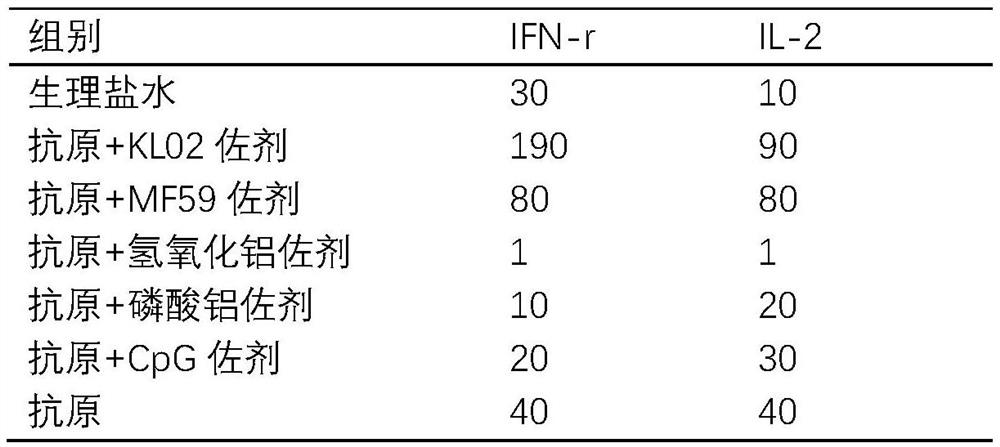
[0050] Example 2. Effect of different adjuvants on the neutralizing antibody level of the new crown vaccine
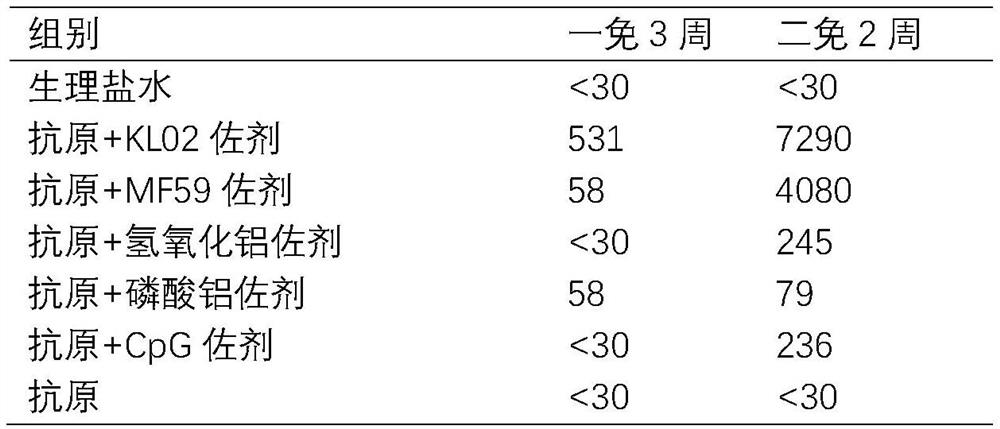
[0051] The HuB strain recombinant protein (see 2020110659137.8 for its preparation method) was mixed with different adjuvant combinations respectively, and mice were immunized. 10 in each group. Each mouse was immunized with 1ug protein in muscle. Booster immunization every two weeks. The blood of the mice was collected three weeks after the first immunization, and the supernatant was collected by centrifugation, and the neutralizing antibody titer of the pseudovirus method and the cytokine detection of the ELISPOT method were carried out. Two weeks after the second immunization, mouse sera were collected for pseudovirus neutralizing antibody detection.
[0052]
[0053] The results showed that in the serum collected two weeks after the second immunization, the neutralizing activity of the KL02 adjuvant and MF59 adjuvant groups was significantly higher than that ...
Embodiment 3
[0056] Embodiment three, the research of antigen dosage
[0057] The recombinant protein of HuB strain (see 2020110659137.8 for its preparation method) was mixed with KL02 adjuvant or aluminum hydroxide adjuvant respectively, and rhesus monkeys were immunized. Two doses were set for the KL02 adjuvant group, 4 monkeys were immunized respectively, and one dose was set for the aluminum hydroxide adjuvant group, a total of 12 rhesus monkeys. A booster immunization was given every 4 weeks for a total of three immunizations. The serum was collected after the second and third immunizations, and the live virus of the new coronavirus HuB strain was used to detect the neutralizing antibody by the new coronavirus live virus neutralizing antibody detection method.
[0058] New coronavirus live virus neutralizing antibody detection method: The cytopathic effect (cytopathic efficiency) detection method is used to detect the neutralizing antibody titer of 50% live virus. Serum samples were...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com