Hypoxia-sensitive drug carrier polymer as well as preparation method and application thereof
A technology of polymers and compounds, which is applied in the field of hypoxia-sensitive polymers and their preparation, can solve the problems of affecting the recognition of DNA transcription points, increasing non-specific adsorption, and reducing transfection efficiency, so as to enhance joint killing ability and enhance hypoxia Oxygen, improve the effect of degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
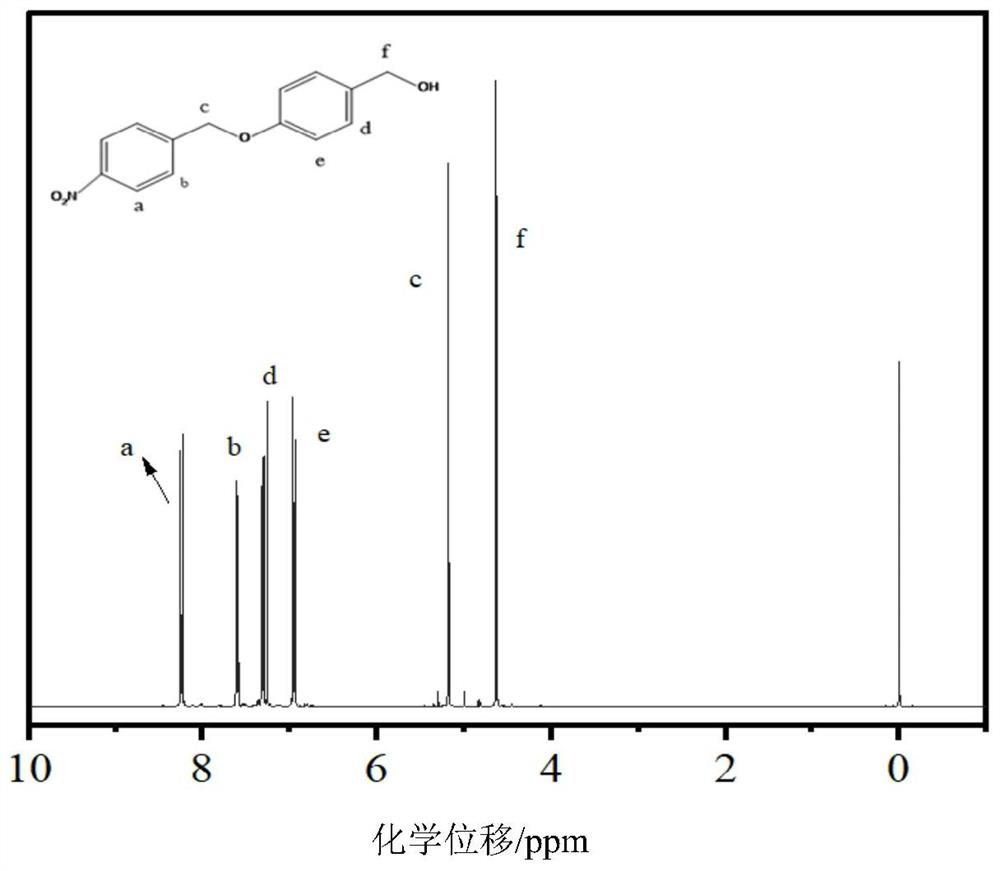
[0083] Embodiment 1: the preparation of compound 4-((4-nitrobenzyloxy)phenyl)methanol
[0084]
[0085] Prepare a 100mL round-bottomed flask with branched mouth, add 3.0g (0.024mol) of solid p-hydroxybenzyl alcohol, 5.52g (0.04mol) of anhydrous potassium carbonate, add 30mL of N,N-dimethylformamide, and stir to make it Hydroxybenzyl alcohol dissolved. Bubble argon and allow to stir under argon for 10 minutes. Dissolve p-nitrobenzyl bromide in 20 mL of N,N-dimethylformamide, and add the solution dropwise into a round-bottomed flask through an atmospheric pressure dropping funnel under argon purging. After the dropwise addition, the solution was light yellow, and after overnight reaction, the solution was dark green. After reacting for 24 hours, remove solid insolubles by filtration, wash the reaction solution with saturated sodium chloride aqueous solution and ethyl acetate successively, and finally collect the organic phase with anhydrous magnesium sulfate to remove water...
Embodiment 2
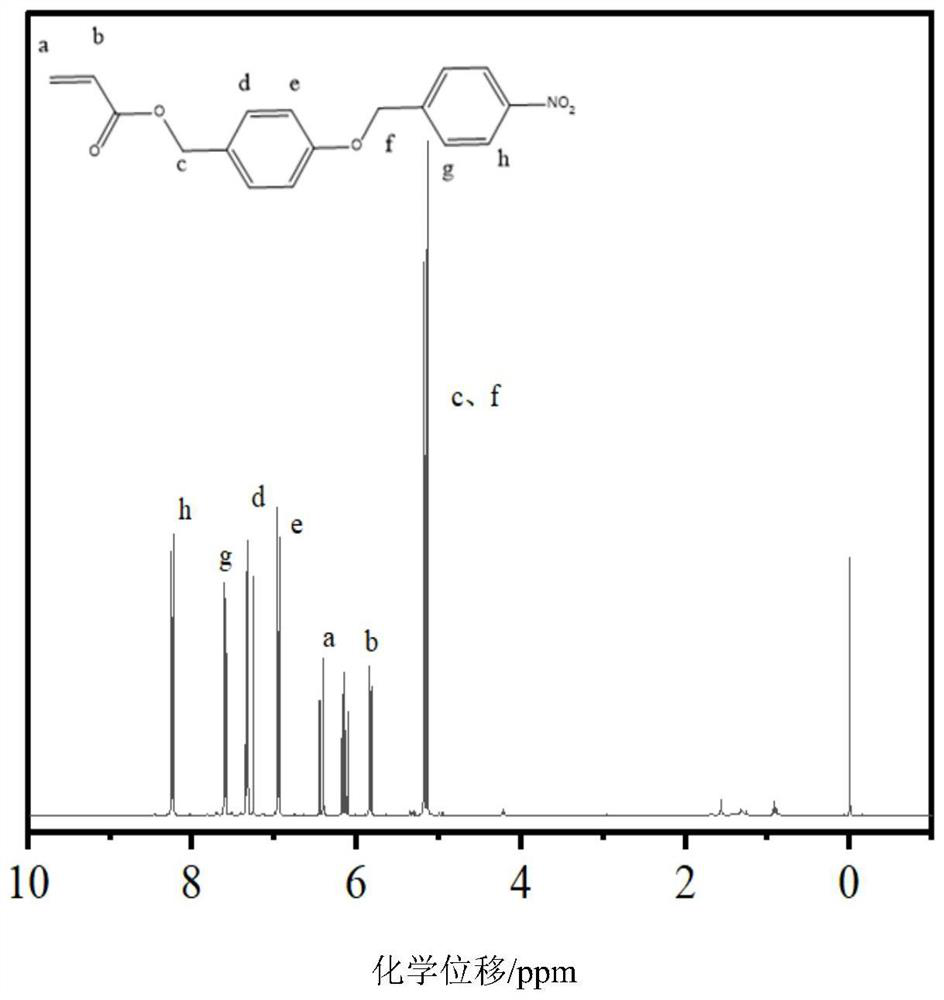
[0086] Embodiment 2: Preparation of 4-((4-nitrobenzyl)oxy)benzyl acrylate
[0087]
[0088] First, weigh 2.6g (0.01mol) of 4-((4-nitrobenzyloxy)phenylcarbinol prepared in Example 1 and dissolve it in 30mL of dichloromethane and add it to a 100mL round-bottomed flask. Add 1mL (1.1mol ) triethylamine as an acid-binding agent, the reaction solution was placed in an ice bath and stirred for 10 minutes so that the temperature of the reaction solution dropped to a lower temperature, and 0.83 mL of acryloyl chloride (0.012 mol) was slowly added dropwise under ice bath conditions, and after overnight reaction Wash with saturated NaCl aqueous solution three times, collect the organic phase, remove water through anhydrous magnesium sulfate, filter to remove the solid, and then evaporate the solvent by rotary evaporation, and separate the product by column chromatography with ethyl acetate:n-hexane volume ratio 1:4, the first point is is the product point. 1 HNMR (CDCl 3 ppm):8.27(m...
Embodiment 3
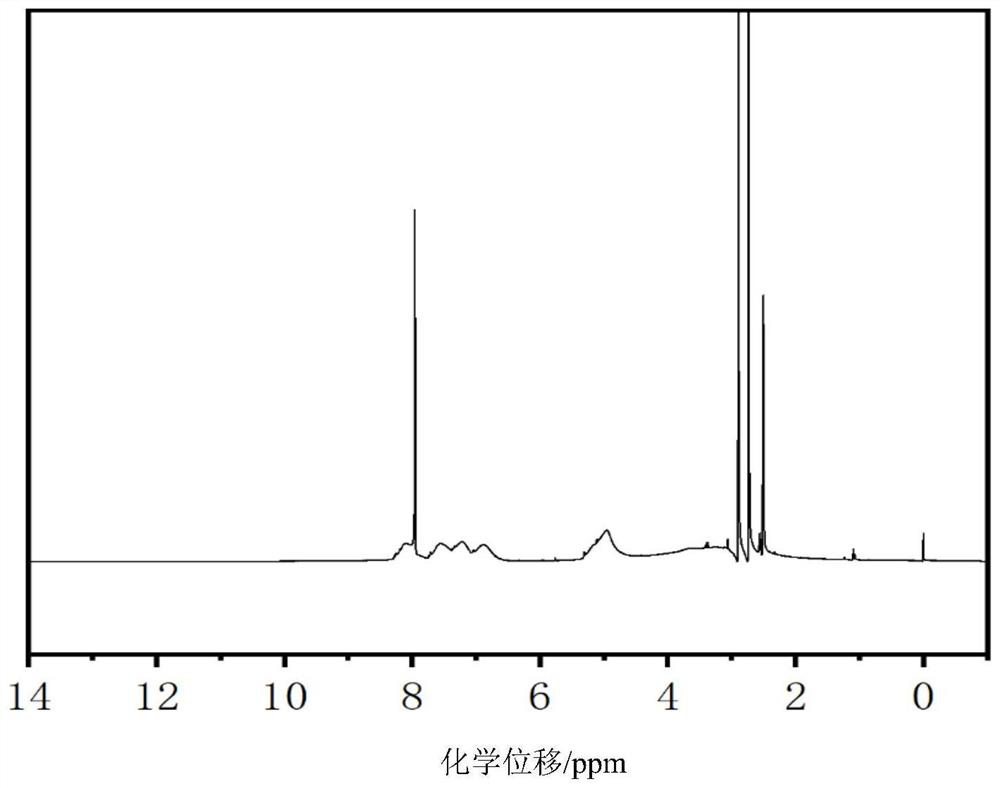
[0089] Example 3: Preparation of Hypoxia Responsive Gene Carrier Polymer A
[0090]
[0091] Accurately weigh 0.1 g of branched polyethyleneimine with a molecular weight of 10K (0.01 mM, containing about 2.3 mM NH for subsequent reaction) into a 5 mL round bottom flask, add 1 mL of anhydrous N, N-dimethylformazine Stir the amide until the polyethyleneimine is completely dissolved. After the dissolution is complete (no obvious viscous liquid), add 1g (3.4mM) of 4-((4-nitrobenzyl)oxy)benzyl acrylate prepared in Example 2 , stirred and reacted at room temperature for 48 hours, and then transferred to a water bath at 45° C. for 24 hours. Add 0.44 mL (7 mM) of iodomethane into the reaction system, and react overnight in the dark. After completion of the reaction, precipitate with ether three times to remove unreacted methyl iodide. After that, dichloromethane was used to precipitate three times to remove unreacted monomers, and the precipitated solid was placed in a vacuum ove...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com