Antigen fusion protein, coding gene and application thereof
A technology of fusion protein and antigen, which is applied in the field of bioengineering, can solve the problems of high fish disease mortality and achieve the effects of easy purification, high yield and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
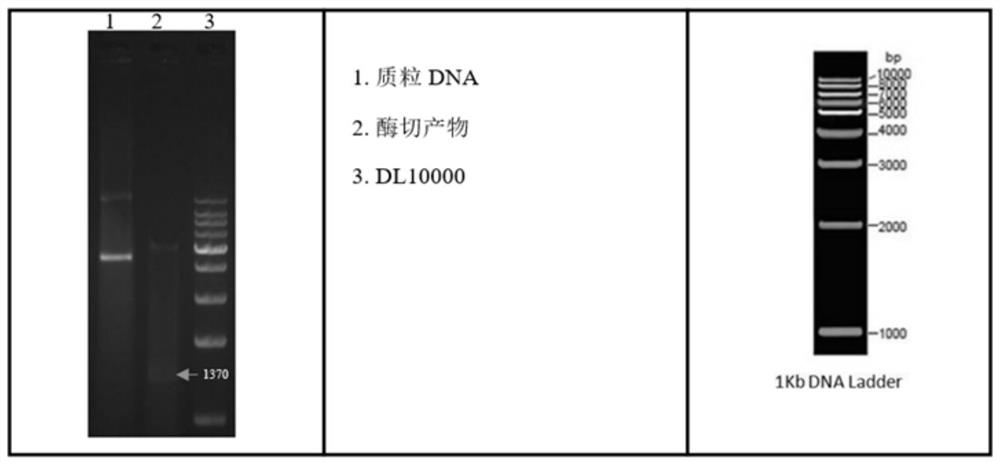
[0035] Example 1 Construction of BL21(DE3)-pET30a-LamB-TloC-OmpN3 expression strain
[0036]According to the sequences of LamB, TolC, and OmpN3 genes reported in GenBank (GenBank accession numbers: Aeromonas victoria-Lamb CP012504.1:c3971056-3969743, Vibrio mimicus-TolC DQ296639.1, ompN3 KJ831564.1), using the epitope prediction online analysis website (http: / / imed.med.ucm.es / Tools / antigenic.pl) to analyze the antigenic determinant regions of the amino acids encoded by the three genes, and select the epitope rich region, LamB (base sequence from 70-450, amino acid sequence from 24-150), TolC (base sequence from 67-567, amino acid sequence from 23-189), OmpN3 (base sequence from 691-1050, amino acid sequence sequence from 231-350). And add 2 linker sequence amino acids (GGGGSGGGGS) in the middle of the fusion gene, add the design restriction enzyme site Nde I (CATATG) at the 5' end, add the design restriction enzyme site Hind III ( AAGCTT).
[0037] The codon optimization so...
Embodiment 2
[0048] Example 2 Massive expression and purification of LamB-TloC-OmpN3
[0049] In the LB liquid medium containing Kana, culture BL21(DE3)-pET30a-LamB-TloC-OmpN3 with shaking at 37°C for about 5-6 hours until the bacterial liquid OD 600 When the value reaches 0.8, add IPTG=0.2mM, induce for 16 hours at 15°C, and collect the bacteria by centrifugation. Co-culture 12L.
[0050] Use 20mM PB (pH7.2), 300mM NaCl, 20mM Imidazole containing 1% Triton X-100, 1mMDTT, 1mM PMSF to lyse the whole bacteria, centrifuge at 12000r / min for 10min at 4°C, separate the soluble part and inclusion body of the expressed protein The dissolved part of the solution was purified by nickel ion column affinity chromatography.
[0051] Supernatant purification: Equilibrate the Ni-IDA affinity chromatography column with 20mM PB (pH7.2), 300mM NaCl, 20mM imidazole buffer, after loading the supernatant, elute the target protein with the equilibration buffer of different concentrations of imidazole, and col...
Embodiment 3
[0054] Example 3 LamB-TloC-OmpN3 quality inspection
[0055] 1. Protein stability test (freeze-thaw experiment)
[0056] Take a piece of LamB-TloC-OmpN3 protein frozen at -80°C, place it in an ice-water bath for 5-10 minutes and wait for it to melt slowly. After melting, place it in a refrigerator at 4°C for 0.5 hours. normal.
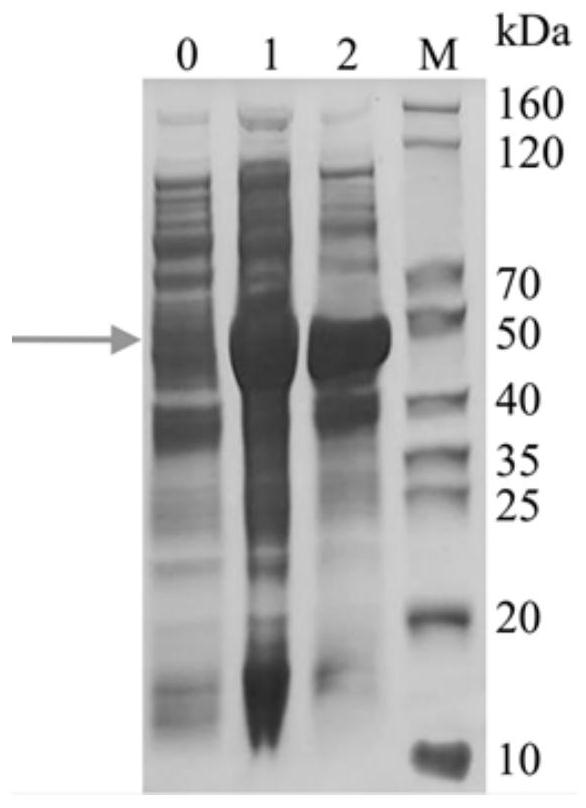
[0057] 2. LamB-TloC-OmpN3 protein concentration and purity determination
[0058] Determination of protein concentration using Bradford protein concentration assay kit, with BSA as a standard, the results show that: protein concentration is 0529mg / mL. According to SDS-PAGE glue R250 staining result shows protein purity > 90% ( Figure 6 ).
[0059] 3. LamB-TloC-OmpN3 protein reactogenicity analysis
[0060] Refer to "Protein Electrophoresis Experimental Technology" written by Guo Yaojun for the operation process of WB experiment, using anti-His as the antibody, the results show that LamB-TloC-OmpN3 is fused with His and can be recognized by the An...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com