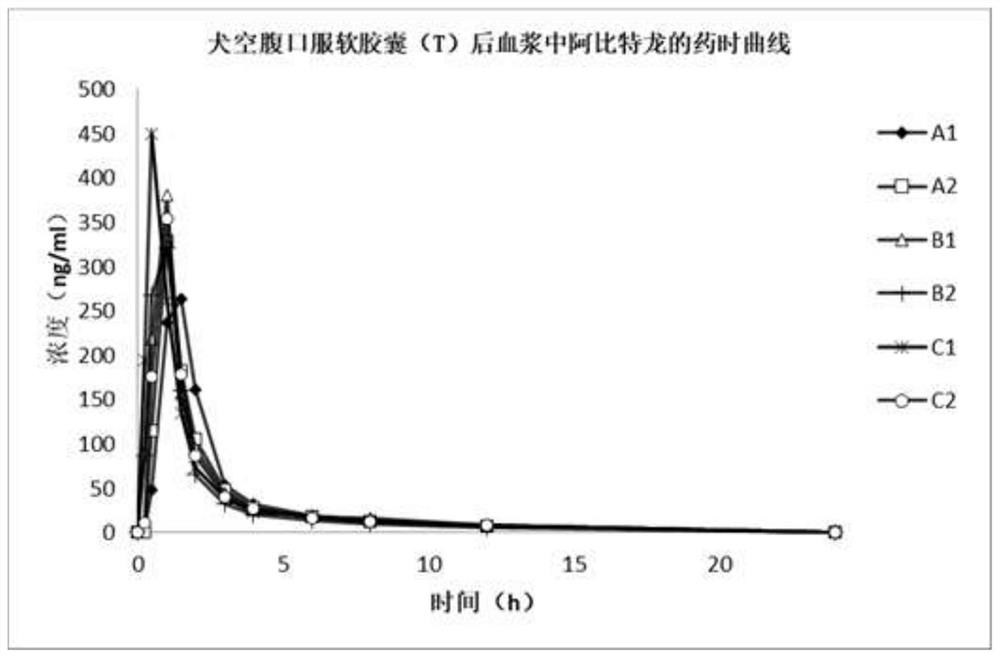
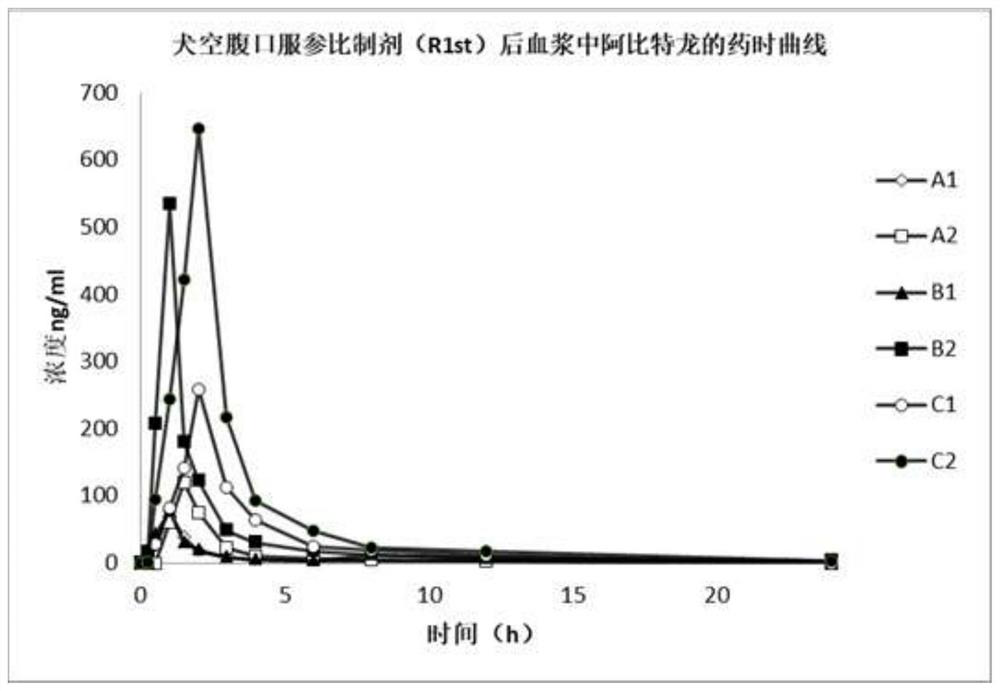
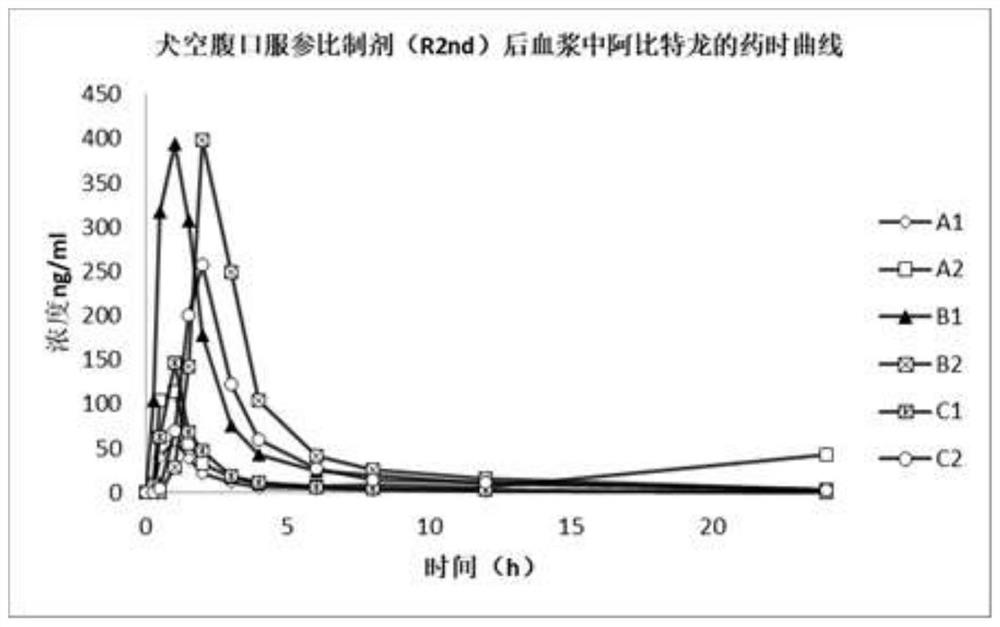
Abiraterone acetate soft capsule and preparation method thereof
A technology of abiraterone acetate and soft capsules, which is applied in capsule delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., and can solve problems such as low solubility, precipitation of abiraterone, and poor oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The present embodiment provides a kind of abiraterone acetate soft capsule (batch: 1000), and its content comprises following component:
[0063]
[0064]
[0065] The above pharmaceutical composition is filled in the soft capsule shell as the content, and the components of the soft capsule shell include: gelatin, water, glycerin, sorbitol and sorbitan solution MDF85, and the ratio of each component is 1:0.74:0.35:0.35.
[0066] The preparation method is provided as follows:
[0067] 1) Chemical glue: Add the prescribed amount of glycerin, sorbitol and water into the chemical glue barrel, start stirring, seal and heat to 75°C, after the materials are evenly mixed, add the prescribed amount of gelatin, and simultaneously vacuumize to -0.05~ -0.06MPa, keep stirring under negative pressure for 25-35min, open the capping respirator until the vacuum disappears, keep warm and degas at 55-60°C, and wait for use; 2) Preparation of contents: Dissolve abiraterone acetate in...
Embodiment 2
[0069] The present embodiment provides a kind of abiraterone acetate soft capsule (batch: 1000), and its content comprises following component:
[0070]
[0071] The above pharmaceutical composition is filled in the soft capsule shell as the content, and the components of the soft capsule shell include: gelatin, water, glycerin, sorbitol and sorbitan solution MDF85, and the ratio of each component is 1:0.74:0.35:0.35.
[0072] The preparation method is provided as follows:
[0073] 1) Chemical glue: Add the prescribed amount of glycerin, sorbitol and water into the chemical glue barrel, start stirring, seal and heat to 75°C, after the materials are evenly mixed, add the prescribed amount of gelatin, and simultaneously vacuumize to -0.05~ -0.06MPa, keep stirring under negative pressure for 25-35min, open the capping respirator until the vacuum disappears, keep warm and degas at 55-60°C, and wait for use; 2) Preparation of contents: Dissolve abiraterone acetate in In the mix...
Embodiment 3
[0075] The present embodiment provides a kind of abiraterone acetate soft capsule (batch: 1000), and its content comprises following component:
[0076]
[0077]
[0078] The above pharmaceutical composition is filled in the soft capsule shell as the content, and the components of the soft capsule shell include: gelatin, water, glycerin, sorbitol and sorbitan solution MDF85, and the ratio of each component is 1:0.74:0.35:0.35.
[0079] The preparation method is provided as follows:
[0080] 1) Chemical glue: Add the prescribed amount of glycerin, sorbitol and water into the chemical glue barrel, start stirring, seal and heat to 75°C, after the materials are evenly mixed, add the prescribed amount of gelatin, and simultaneously vacuumize to -0.05~ -0.06MPa, keep stirring under negative pressure for 25-35min, open the capping respirator until the vacuum disappears, keep warm and degas at 55-60°C, and wait for use; 2) Preparation of contents: Dissolve abiraterone acetate in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com