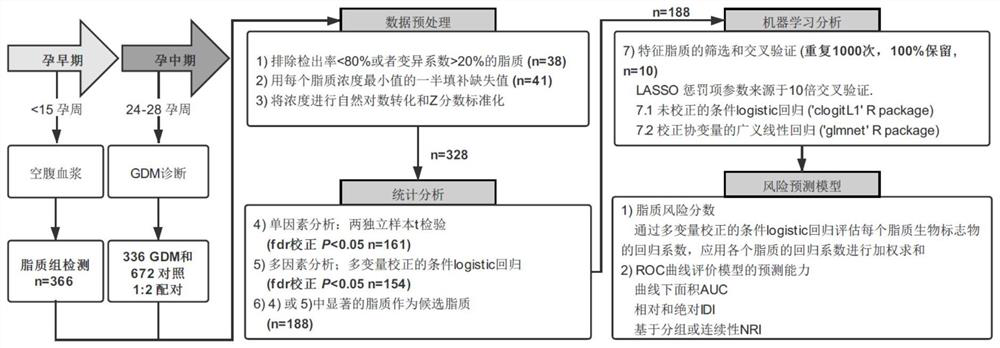
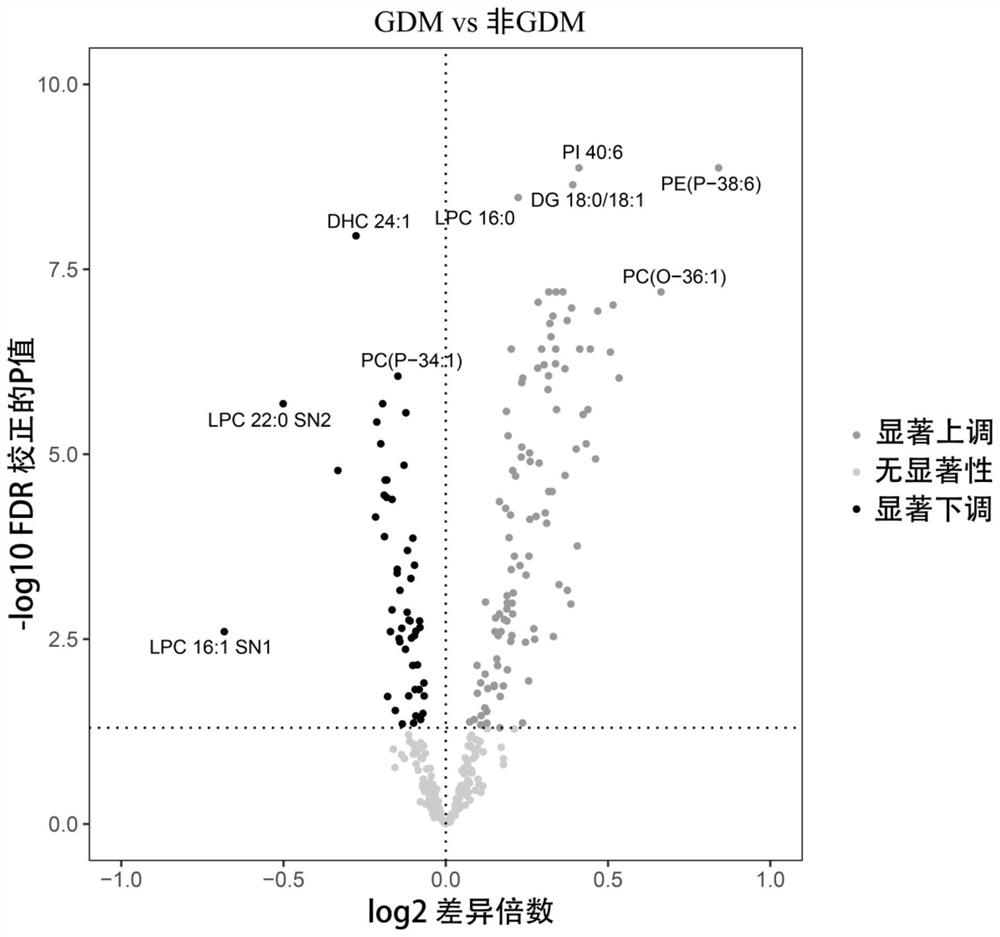
Screening and application of early pregnancy blood lipid biomarker for gestational diabetes mellitus
A biomarker and diabetes technology, applied in biological testing, biomaterial analysis, disease diagnosis, etc., can solve the problems of establishing prediction models, unclear time series relationship, small sample size, etc., achieve comprehensive screening steps, improve prediction efficiency, The effect of rigorous screening steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1: extract plasma lipid
[0076] Aliquot 20 μL of plasma to 11 μL of internal standard mixture (containing 400 pmol PC 14:0, PE 17:0, CE16:0(d7), COH(d7), BMP 14:0, SM 12:0, PG 14:0 and PA 14:0; and 50 pmol TG 16:0 / 18:0 / 16:0(d5), LC 16:0(d3), GC 16:0(d3)], continue with 244 μL butanol: A mixture of methanol (1:1, v / v) and 10 mM ammonium formate was mixed for lipid extraction and protein precipitation. The sample mixture was fully vortexed and sonicated in ice water for 1 hour, and centrifuged at 14000×g for 10 min at 20 ° C. The supernatant Transfer to instrumental analysis.
Embodiment 2
[0077] Example 2: Ultra-high performance liquid chromatography-mass spectrometry on-board detection
[0078] Using ExionLCTM AD ultra-high performance liquid chromatography-mass spectrometry (UHPLC, AB Sciex, Toronto, Canada), equipped with Agilent Eclipse Plus C18 column (Agilent Technologies), and combined with QTRAP 5500 mass spectrometer (AB Sciex, Toronto). A ZORBAX Eclipse Plus C18 column (1.8 μm, 2.1×50 mm, Agilent) was used according to the chromatographic conditions, and the sample injection volume was 2 uL. Mobile phase A and mobile phase B were mixtures of water, acetonitrile and isopropanol in ratios of 50:30:20 and 1:9:90, respectively, and both A and B contained 10 mM ammonium formate. Control the autosampler at 25°C and control the temperature of the chromatographic column at 45°C to achieve optimal analyte solubility and peak separation. The mobile phase flow rate was 0.3 ml / min and the gradient was as follows: start with 25% B, increase from 25% B to 49% in 0...
Embodiment 3
[0085] Embodiment 3: the processing of chromatogram
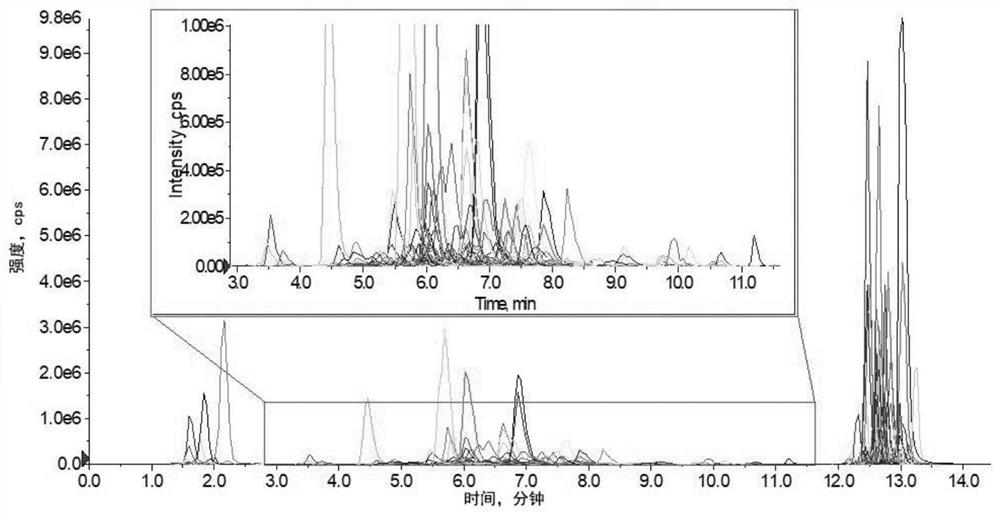
[0086] Import the experimental data into the analysis1.6.3 software (AB Sciex), and draw the standard curve. The chromatogram of the plasma lipid group is attached figure 2 shown. The concentration of each molecule was calculated using the internal standard method by relating the peak area of each molecule to that of the corresponding internal standard.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com