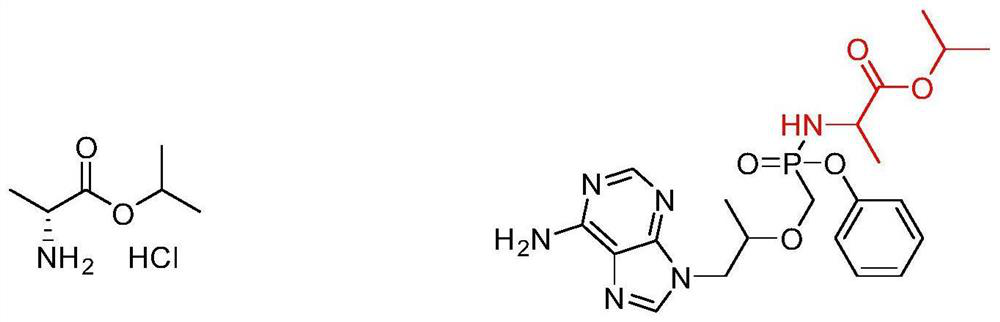
Method for determining related substances of L-alanine isopropyl ester hydrochloride by adopting high performance liquid chromatography
A technology of isopropyl alanine and high-performance liquid chromatography, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as the determination method of L-isopropyl alanine hydrochloride that has not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The selection of embodiment 1 chromatographic conditions
[0039] 1.1 Selection of wavelength
[0040] Diluent: 25% acetonitrile in water.
[0041] Preparation of L-alanine isopropyl ester hydrochloride solution: Weigh about 10mg of L-alanine isopropyl ester hydrochloride, put it in a 10ml measuring bottle, add diluent to dissolve, dilute to the mark, shake well, Instantly.
[0042] Take L-alanine isopropyl ester hydrochloride solution as the test sample, use the diluent as the blank calibration solution, and scan at 190nm to 400nm according to the ultraviolet-visible spectrophotometry (Chinese Pharmacopoeia 2020 edition). The result shows that need testing solution has maximum absorption peak at 205nm. Therefore, 205nm±2nm was determined as the detection wavelength.
[0043] 1.2 Durability test of the method
[0044] In this test, the chromatographic method of this test is screened by adjusting the mobile phase ratio, flow rate, column temperature, etc. See Table...
Embodiment 2
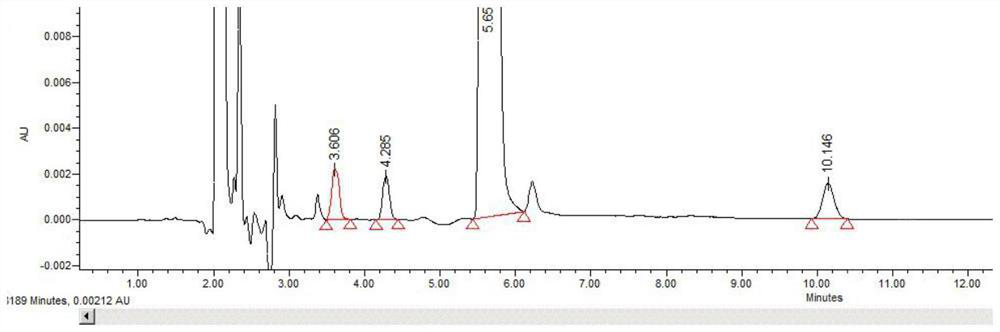
[0057] Example 2 Specificity
[0058] Chromatographic conditions: the same as in Example 1, scheme 1.
[0059] Solution preparation:
[0060] Blank solution: diluent
[0061] Acid destruction blank solution: Add 2.5ml of 0.1mol / L hydrochloric acid solution to a 5ml measuring bottle, let it stand at room temperature for 4 hours; dilute with 0.1mol / L sodium hydroxide to the mark, shake well, and obtain.
[0062] Acid-breaking test solution: Accurately weigh about 50 mg of the test product, place it in a 5ml measuring bottle, add 2.5ml of 0.1mol / L hydrochloric acid solution, let it stand at room temperature for 4h; dilute to the mark with 0.1mol / L sodium hydroxide, Shake well, and you get it.
[0063] Alkali destruction blank test solution: add 2.5ml of 0.1mol / L sodium hydroxide solution to a 5ml measuring bottle, let it stand at room temperature for 4h; dilute with 0.1mol / L hydrochloric acid to the mark, shake well, and obtain.
[0064] Alkali destruction of the test product...
Embodiment 3
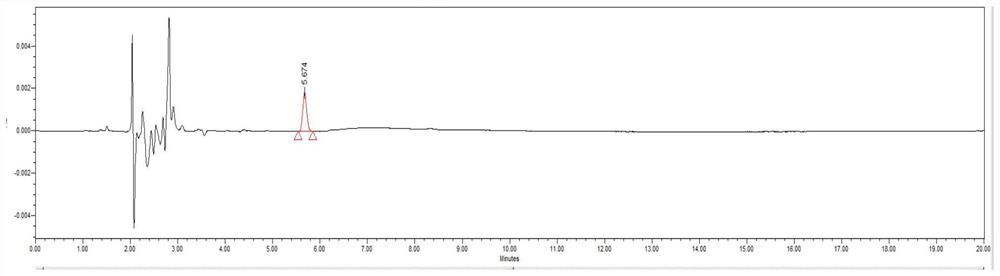
[0076] The stability of embodiment 3 solution
[0077] Chromatographic conditions: the same as in Example 1, Scheme 1.
[0078] Solution preparation:
[0079] Need testing solution: prepare by the same method of 100% concentration level need testing solution in embodiment 1;
[0080] Control solution: accurately pipette 1ml of the test solution, put it in a 100ml measuring bottle, dilute to the mark with diluent, shake well, then precisely pipette 1ml, put it in a 10ml measuring bottle, dilute to the mark with diluent as diluent, shake well , that is.
[0081] Respectively at 0 hour, 12 hours, 24 hours, 48 hours, 10 μl of the test solution and control solution were precisely measured, and the chromatograms at different time points were recorded. The results are shown in Table 4.
[0082] Table 4 solution stability result
[0083]
[0084] The results showed that both the test solution and the control solution were stable within 48 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com