An organic material capable of absorbing near-infrared light and its preparation method and application
A technology of organic materials and near-infrared light, applied in organic chemistry, optical filters, etc., can solve the problems of low absorption efficiency of near-infrared light, unfavorable applications, and limited use range, etc., and achieve good near-infrared light absorption performance, charge push-pull Effect enhancement, effect that promotes rapid dissipation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation steps of the organic material that can absorb near-infrared light in this example are as follows:
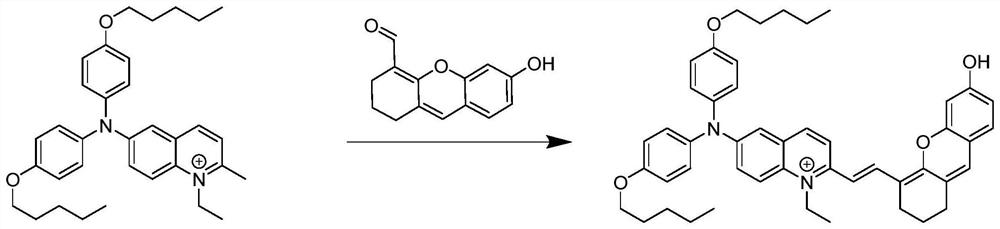
[0031] Take 319.12 mg of 6-(bis(4-(pentyloxy)phenyl)amino)-1-ethyl-2-methylquinoline salt (0.50 mmol) containing iodine counterion and 114.04 mg of 6-hydroxy-2 , 3-Dihydroxanthene-4-carbaldehyde (0.50 mmol) was added to a one-necked flask, and 5 mL of n-butanol was added and mixed evenly. The mixed solution was heated to 118°C, stirred and refluxed for 10h. After the reaction was finished, it was lowered to room temperature, and the organic solvent was removed by rotary evaporation, and the resulting solid was purified by silica gel column chromatography (the eluent used was dichloromethane / ethyl acetate, V / V=20:1) to obtain the product 6- (Bis(4-(pentyloxy)phenyl)amino)-1-ethyl-2-(2-(6-hydroxy-2,3-dihydroxanthene)vinyl)quinoline salt.
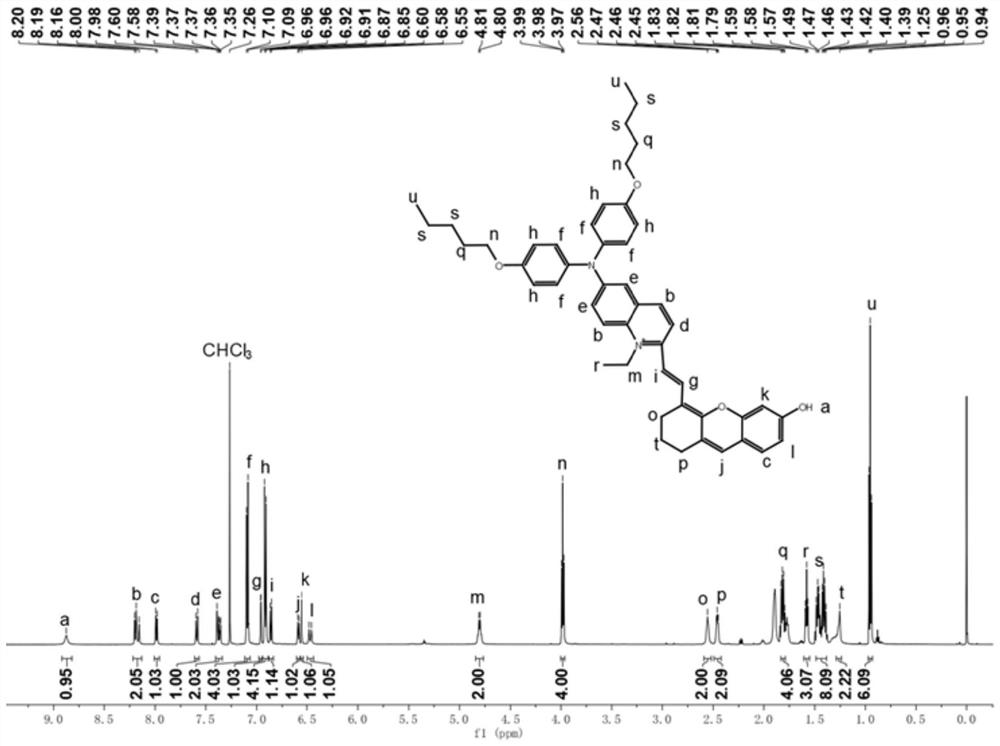
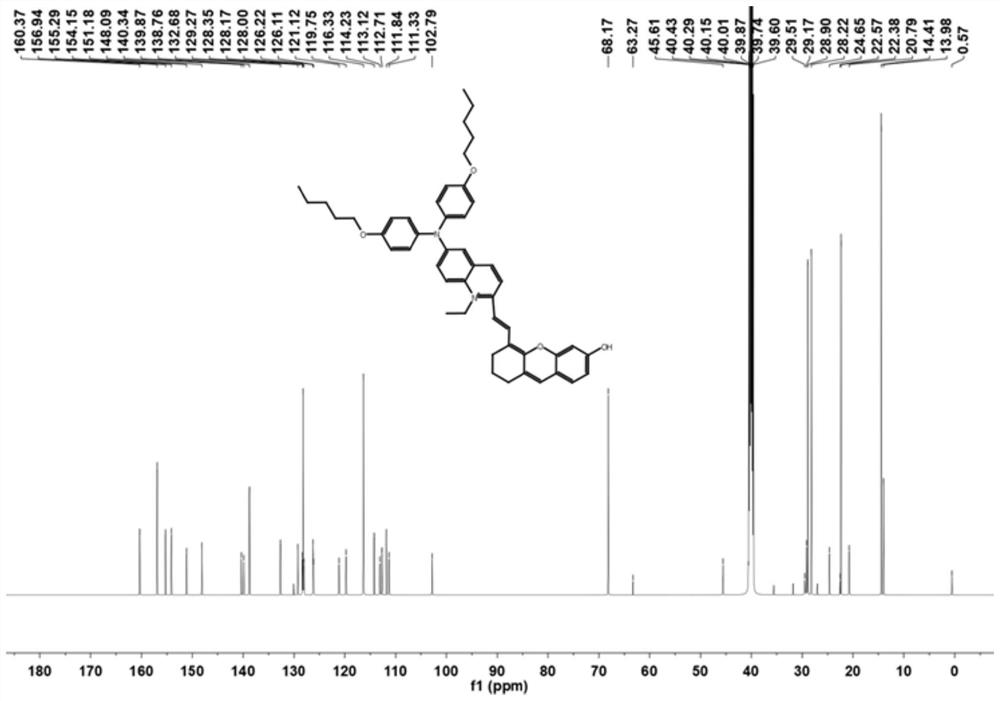
[0032] The resulting product was characterized by proton nuclear magnetic resonance spectroscopy: 1 H NMR (600MHz, CD...
Embodiment 2
[0036] The preparation steps of the organic material that can absorb near-infrared light in this example are as follows:
[0037] Take 1.276 g of 6-(bis(4-(pentyloxy)phenyl)amino)-1-ethyl-2-methylquinolinate (2.00 mmol) containing iodine counterion and 547.39 mg of 6-hydroxy - 2,3-Dihydroxanthene-4-carbaldehyde (2.40 mmol) was added to a one-necked flask, and 24 mL of n-butanol was added and mixed evenly. The mixed solution was heated to 122°C, stirred and refluxed for 12h. After the reaction was finished, it was lowered to room temperature, and the organic solvent was removed by rotary evaporation, and the resulting solid was purified by silica gel column chromatography (the eluent used was dichloromethane / ethyl acetate, V / V=20:1) to obtain the product 6- (Bis(4-(pentyloxy)phenyl)amino)-1-ethyl-2-(2-(6-hydroxy-2,3-dihydroxanthene)vinyl)quinoline salt.
[0038] The final product characterization of Example 2 is the same as the result in Example 1.
Embodiment 3
[0040] The preparation steps of the organic material that can absorb near-infrared light in this example are as follows:
[0041] Take 638.24 mg of 6-(bis(4-(pentyloxy)phenyl)amino)-1-ethyl-2-methylquinolinate (1.00 mmol) containing iodine counterion and 250.89 mg of 6-hydroxy -2,3-Dihydroxanthene-4-carbaldehyde (1.10 mmol) was added to a one-necked flask, and 11 mL of n-butanol was added and mixed evenly. The mixed solution was heated to 120°C, stirred and refluxed for 11h. After the reaction was finished, it was lowered to room temperature, and the organic solvent was removed by rotary evaporation, and the resulting solid was purified by silica gel column chromatography (the eluent used was dichloromethane / ethyl acetate, V / V=20:1) to obtain the product 6- (Bis(4-(pentyloxy)phenyl)amino)-1-ethyl-2-(2-(6-hydroxy-2,3-dihydroxanthene)vinyl)quinoline salt.
[0042] The final product characterization of Example 3 is the same as the result in Example 1.
[0043] The 6-(bis(4-(pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com