Novel organic material capable of absorbing near-infrared light as well as preparation method and application thereof
A technology of near-infrared light and organic materials, applied in organic chemistry, chemical instruments and methods, and other chemical processes, can solve problems such as weak absorption effects, cumbersome and complex compound synthesis processes, and narrow absorption bands, and achieve enhanced charge push-pull effects , Low production cost, and the effect of promoting absorption and transformation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
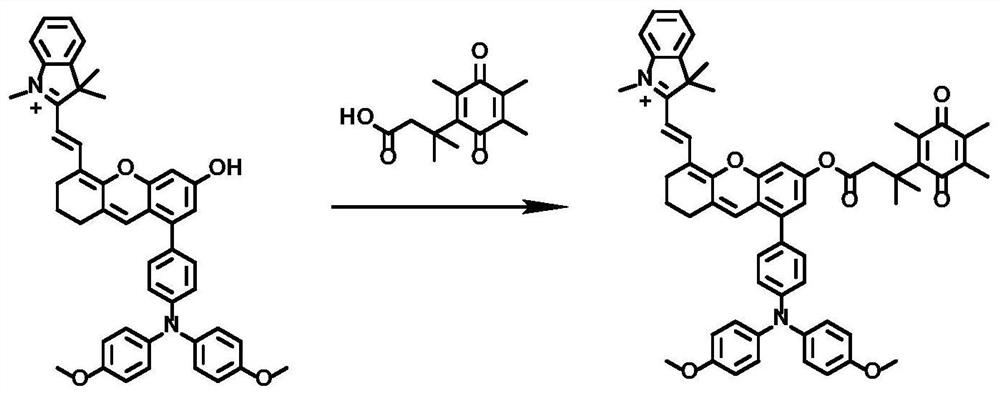
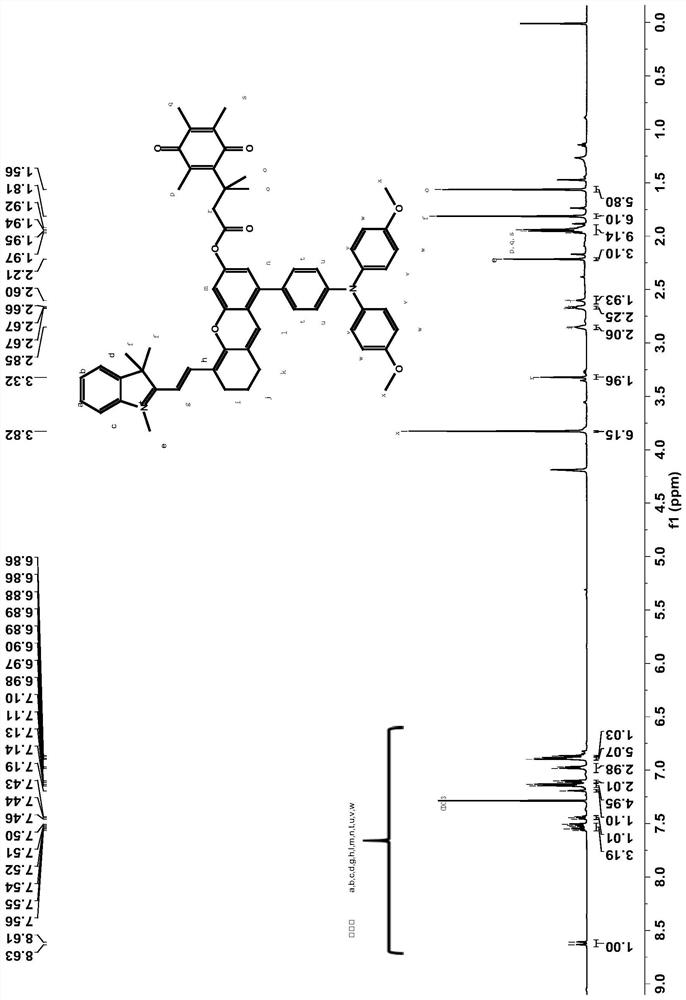
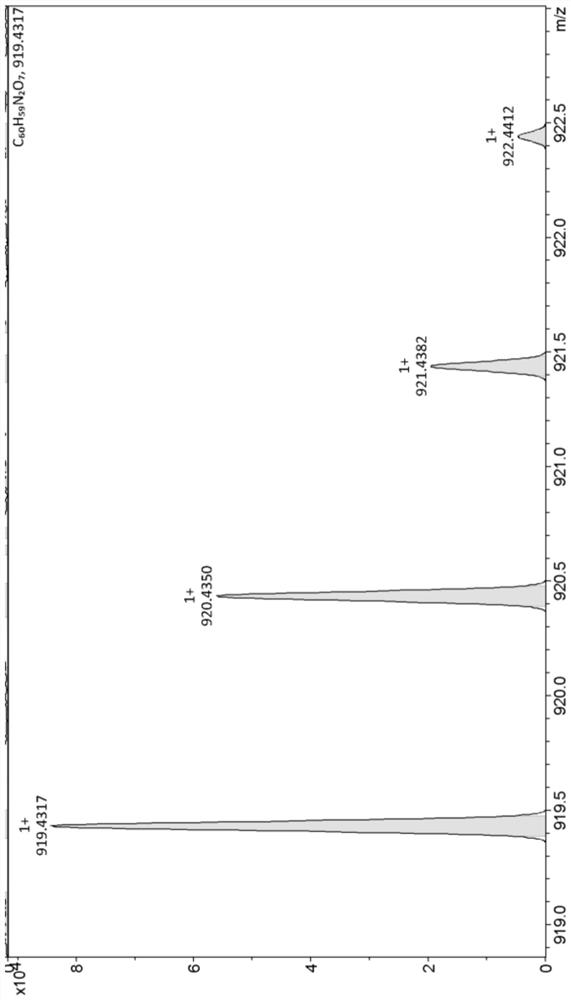
[0035] Take 344.00 mg of 2-(2-(8-(4-(bis(4-methoxyphenyl)amino)phenyl)-6-hydroxy-2,3-dihydroxanth-4-yl) Vinyl)-1,3,3-trimethylindole salt (0.50mmol) and 125.27mg of 3,3-dimethyl-3-(2,4,5-trimethyl-3,6-di Oxycyclohexa-1,4-dien-1-yl)propionic acid (0.50mmol) was dissolved in a one-necked flask by adding 3mL of dichloromethane to obtain solution 1, which was cooled to 0°C. Dissolve 206.53mg of dicyclohexylcarbodiimide (1.00mmol) and 6.11mg of 4-dimethylaminopyridine (0.05mmol) in 3mL of dichloromethane to obtain solution 2, and inject solution 2 under nitrogen protection with a syringe Added dropwise to solution 1. The mixed solution was heated to room temperature and the reaction was stirred for 16h. Add water and dichloromethane for extraction, take the organic phase, dry with anhydrous sodium sulfate, and filter; remove the organic solvent by rotary evaporation, and the resulting solid is purified by silica gel chromatography (the eluent used is dichloromethane / methanol, V / V...
Embodiment 2
[0039] Take 1.511 g of 2-(2-(8-(4-(bis(4-methoxyphenyl)amino)phenyl)-6-hydroxy-2,3-dihydroxanth-4-yl) Vinyl)-1,3,3-trimethylindole salt (2.20mmol) and 660.28mg of 3,3-dimethyl-3-(2,4,5-trimethyl-3,6-di Oxycyclohexa-1,4-dien-1-yl)propionic acid (2.64mmol) was dissolved in a one-necked flask by adding 21.98mL of dichloromethane to obtain solution 1, which was cooled to 2°C. Dissolve 997.91mg of dicyclohexylcarbodiimide (4.84mmol) and 53.72mg of 4-dimethylaminopyridine (0.44mmol) in 24.18mL of dichloromethane to obtain solution 2, and use a syringe to place solution 2 under nitrogen protection Added dropwise to solution 1. The mixed solution was heated to room temperature and the reaction was stirred for 18h. Add water and dichloromethane for extraction, take the organic phase, dry with anhydrous sodium sulfate, and filter; remove the organic solvent by rotary evaporation, and the resulting solid is purified by silica gel chromatography (the eluent used is dichloromethane / metha...
Embodiment 3
[0042] Take 687.00 mg of 2-(2-(8-(4-(bis(4-methoxyphenyl)amino)phenyl)-6-hydroxy-2,3-dihydroxanth-4-yl) Vinyl)-1,3,3-trimethylindole salt (1.00mmol) and 275.19mg of 3,3-dimethyl-3-(2,4,5-trimethyl-3,6-di Oxycyclohexa-1,4-dien-1-yl)propionic acid (1.10mmol) was dissolved in a one-necked flask by adding 7.5mL of dichloromethane to obtain solution 1, which was cooled to 5°C. Dissolve 433.09mg of dicyclohexylcarbodiimide (2.10mmol) and 18.32mg of 4-dimethylaminopyridine (0.15mmol) in 8.4mL of dichloromethane to obtain solution 2, and use a syringe to protect solution 2 under nitrogen Add dropwise to a one-necked flask. The mixed solution was heated to room temperature and the reaction was stirred for 17h. Add water and dichloromethane for extraction, take the organic phase, dry with anhydrous sodium sulfate, and filter; remove the organic solvent by rotary evaporation, and the resulting solid is purified by silica gel chromatography (the eluent used is dichloromethane / methanol, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com