Classical swine fever virus E2 protein recombinant subunit vaccine taking salmonella flagellin as molecular adjuvant and preparation method of classical swine fever virus E2 protein recombinant subunit vaccine
A subunit vaccine and flagellin technology are applied in the field of swine fever virus E2 protein recombinant subunit vaccine and its preparation, and can solve the problems of inability to produce IgA antibodies and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
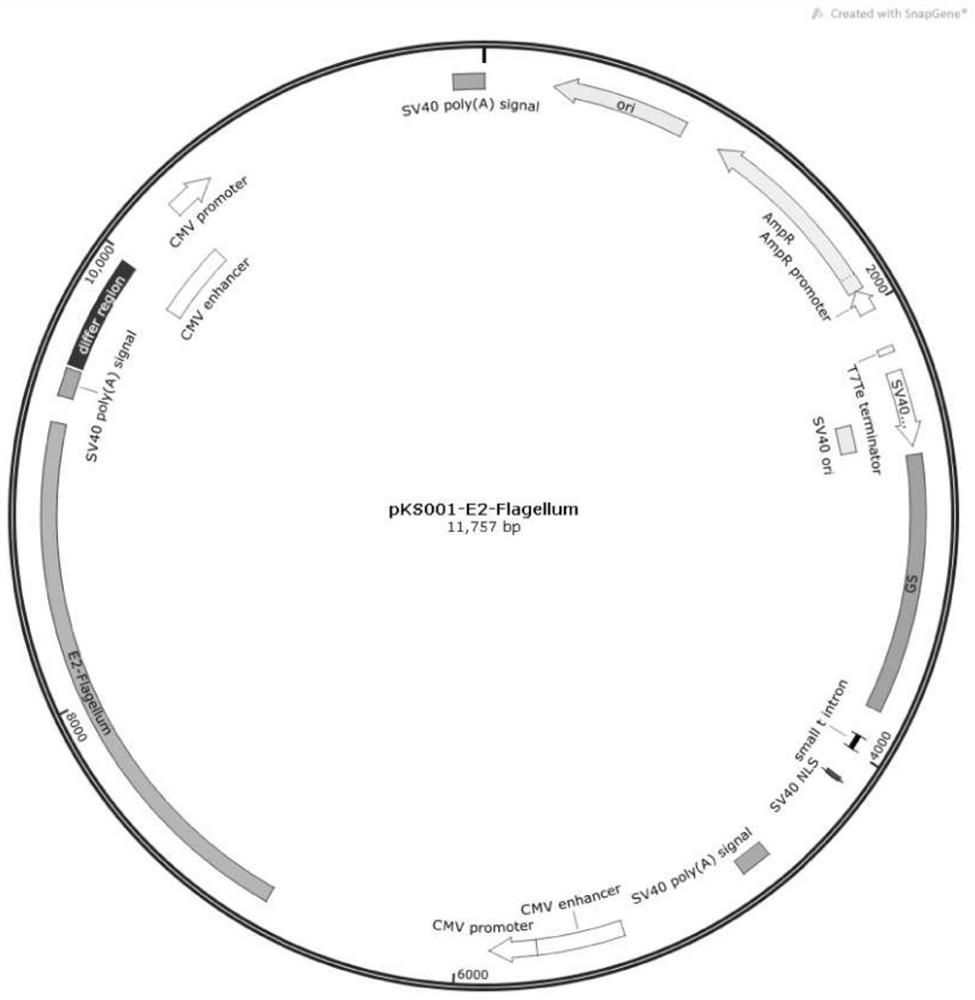
[0033] Example 1 Expression vector construction and protein preparation
[0034]The present invention is based on the E2 protein gene of CSFV 2.1 strain (GenBank accession: JX262391) and the flagellin Flagellum gene of Enterica serovar Typhimurium strain (GenBank accession: CP007581.1), and designs the combination of E2 and Flagellum protein Fusion expression sequence E2-Flagellum (sequence as shown in SEQ ID NO.1, 2373bp in total), wherein E2 has removed the transmembrane region of the C-terminus, and the D3 region of the Flagellum protein is carried out with the (P2) T cell epitope QYIKANSKFIGITEL of tetanus toxin Instead, a rigid Linker (EAAAK) was used between E2 and Flagellum, and a 6×HIS tag was added to the tail of the fusion protein. The final gene sequence was sent to Nanjing GenScript Biotech Co., Ltd. for codon-optimized synthesis.
[0035] PCR amplification primers were designed, and the synthetic E2-Flagellum gene fragment was used as a template to amplify the E2-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Passage density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com