Tumor-targeted multifunctional non-viral gene vector and preparation method and application thereof
A gene carrier and tumor targeting technology, applied in the field of medicine, can solve the problems of not fully meeting the clinical needs of gene therapy, unsatisfactory transfection effect, and low transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
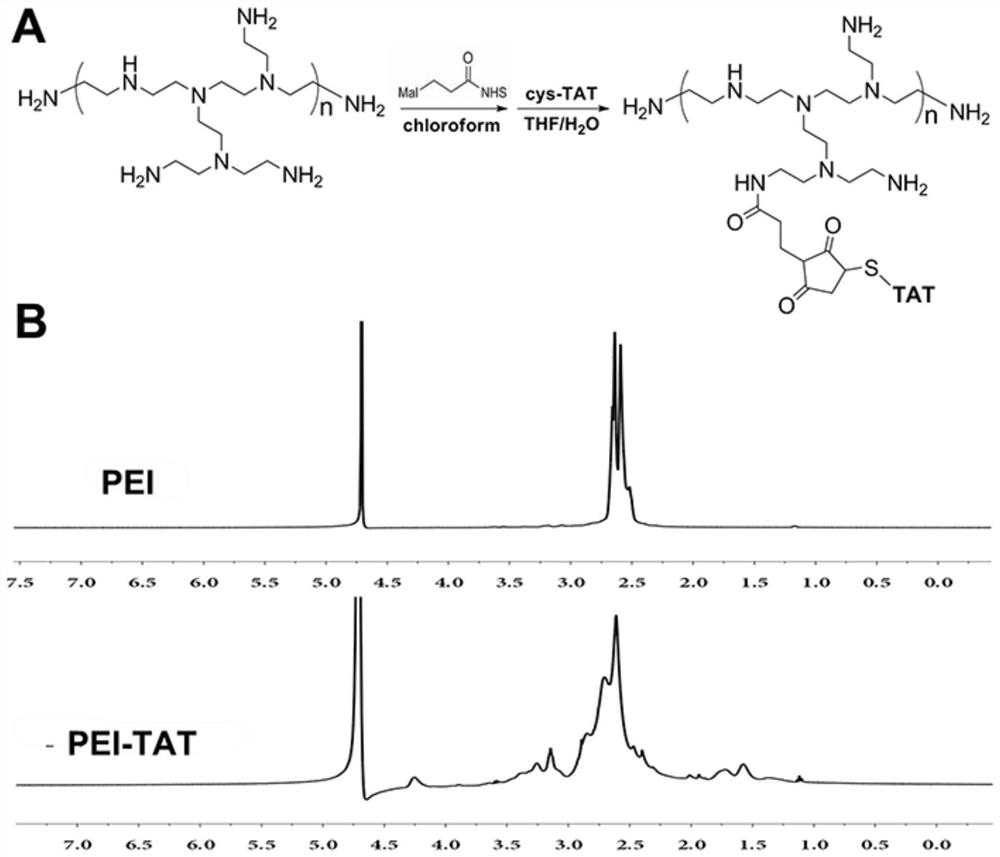
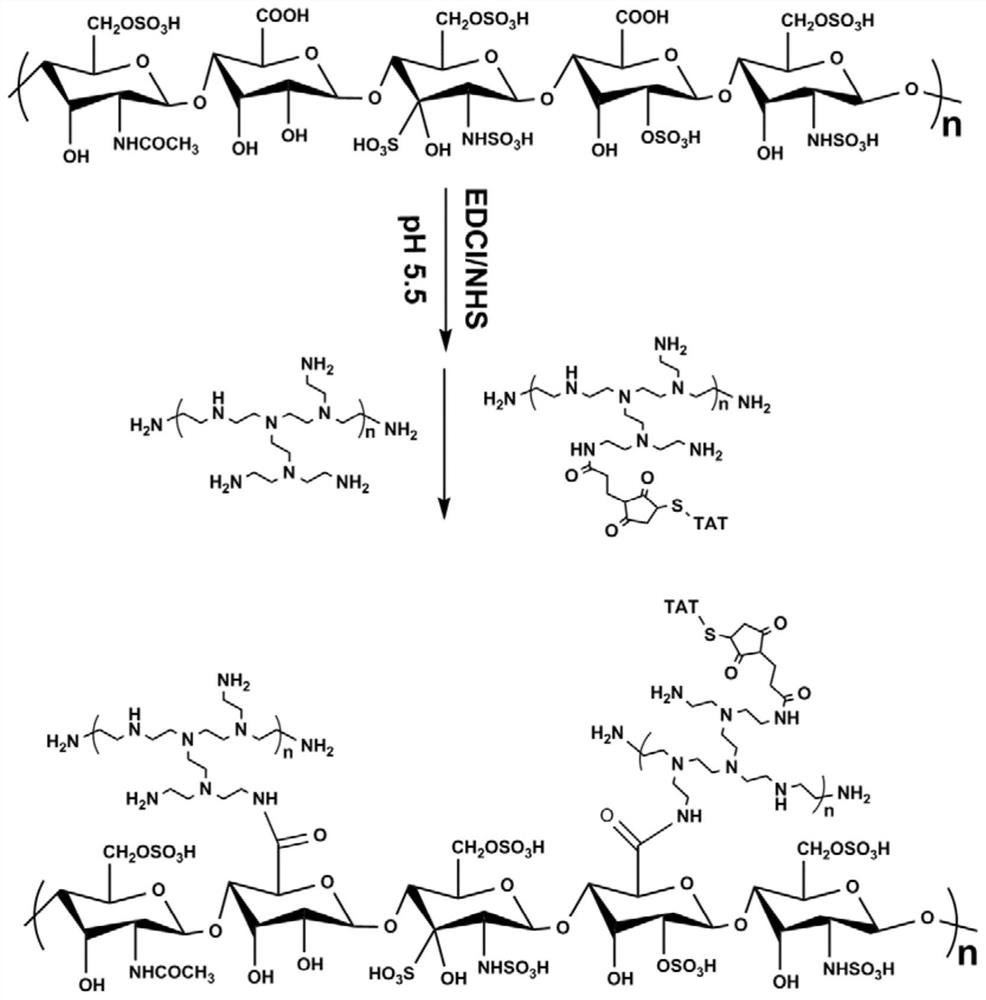
[0086] Embodiment 1, the cationic core of the non-viral gene carrier (HPTHPU nanoparticle) of the present invention: preparation of heparin-PEI-TAT (HPT) nanoparticle
[0087] (1) The synthesis method of PEI-TAT is as follows: first, PEI (500 mg, 0.275 mol, 1.0 eq) with a molecular weight of 1800 was dissolved in 10 mL of chloroform, and then 3-maleimidopropionic acid hydroxysuccinimide The ester (88.5mg, 0.333mmol, 1.2eq) was added to the above solution, and after reacting at room temperature for 3 hours, the reaction solvent, chloroform, was removed by rotary evaporation. The remaining residue was fully dissolved with a mixed solution of 10 mL tetrahydrofuran (THF) and water (the volume ratio of THF and water was 1:1), and then the penetrating peptide Cys-TAT (457 mg, 0.275 mmol, 1.0 eq) was added to the above solution The reaction was continued at room temperature for 6 hours. After the reaction was finished, the reaction solution was dialyzed in pure water for 3 days with...
Embodiment 2
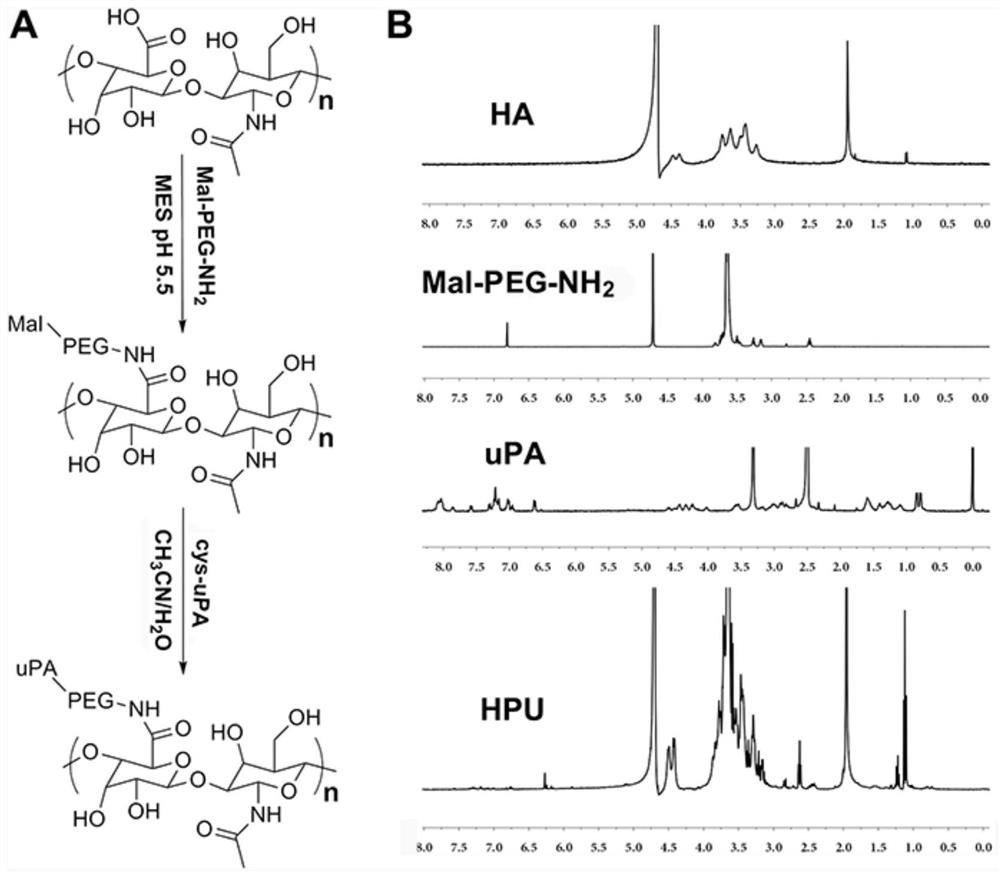
[0092] Embodiment 2, the anionic shell of the non-viral gene carrier (HPTHPU nanoparticle) of the present invention: the preparation of HA-PEG-uPA (HPU)
[0093] Hyaluronic acid (HA, 100mg), EDCI (14.2mg) and NHS (8.5mg) were dissolved together in 30mL MES buffer (pH 5.5, 0.05M), and reacted at room temperature for 2h to fully activate the carboxyl groups on HA. Then Mal-PEG-NH 2 (weight-average molecular weight: 2000, 124 mg) was added to the above reaction solution and continued to react for 24 hours at room temperature. Subsequently, the reaction solution was transferred to a dialysis bag with a molecular weight cut-off of 3500, and then dialyzed in pure water for 3 days to remove unreacted impurities. After dialysis, the liquid in the dialysis bag was reacted with cys-uPA (7.44mg, 0.0124069mmol) in 10mL of THF and water mixed solution (the volume ratio of THF and water was 1:1) for 48h. Finally, the above reaction solution was dialyzed in pure water for 3 days with a dia...
Embodiment 3
[0095] Embodiment 3, the preparation method (not carrying plasmid DNA) when the non-viral gene carrier (HPTHPU nanoparticle) of the present invention is used
[0096] The HPU powder prepared in Example 2 was dissolved in water to obtain an HPU aqueous solution. Then, the solution containing HPT nanoparticles prepared in Example 1 and the HPU aqueous solution were fully mixed according to the mass ratio of HPU powder and HPT nanoparticles at 3:1, and then incubated at room temperature for 25 minutes to obtain HPTHPU nanoparticles.
[0097] According to the preparation method described in Example 3, only by changing the mass ratio of HPT nanoparticles and HPU powder, HPTHPU nanoparticles with different mass ratios of HPT nanoparticles and HPU powder can be obtained. The mass ratio of HPU powder and HPT nanoparticles can also be 0.5:1, 1:1, 1.5:1, 2:1, 2.5:1, 3.5:1, 4:1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com