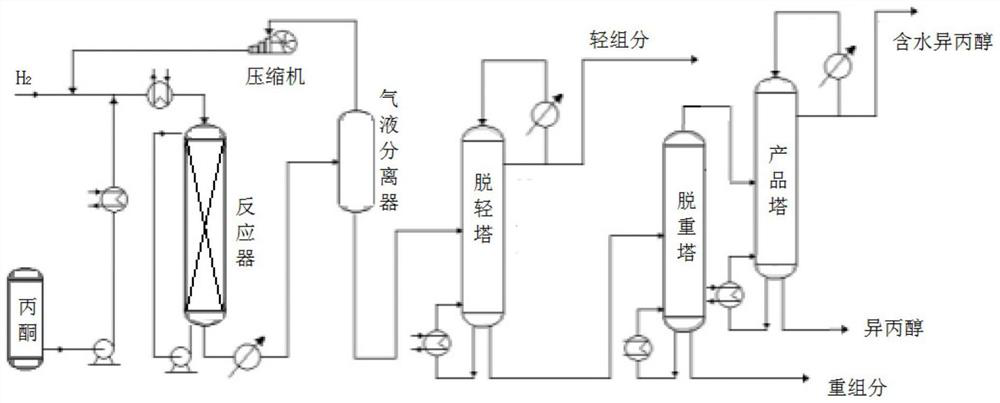
Method for producing isopropanol
A technology of isopropanol and acetone, applied in the production field of isopropanol, can solve the problems of high investment, high energy consumption and high purity of isopropanol products, and achieves low investment, low process energy consumption and good heat exchange effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Fill the adiabatic fixed-bed reactor with 12mL catalyst and pass through H 2 After three replacements, use a metering pump to inject liquid acetone into the preheater and heat it to 70°C, then drop it into the reactor from the top of the reactor, and react in H2 2 Under the following conditions, the reaction pressure is 1.5MPa, the reactor inlet temperature is 100°C, and the liquid space velocity is 1.2h -1 , the molar ratio of acetone to hydrogen is 1:10, and the reaction product is separated to obtain the product isopropanol through light removal, weight removal and dehydration. The purity of the product isopropanol is 99.93%, and the single-pass conversion rate of acetone is 98.2%. The property is 99.2% based on acetone.
Embodiment 2
[0034] Fill 12mL catalyst into the adiabatic fixed-bed reactor, pass H 2 After three replacements, use a metering pump to inject liquid acetone into the preheater and heat it up to 60°C, then drop it into the reactor from the top of the reactor, and react in H2 2 Under the following conditions, the reaction pressure is 2.5MPa, the reactor inlet temperature is 120°C, and the liquid space velocity is 0.8h -1 , the acetone-hydrogen molar ratio is 1:5, and the reaction product is separated by light removal, weight removal and dehydration to obtain the product isopropanol, with a purity of 99.92%, a single-pass conversion rate of acetone of 98.5%, and the selectivity of isopropanol calculated by acetone as 99.7% .
Embodiment 3
[0036] Fill 12mL catalyst into the adiabatic fixed-bed reactor, pass H 2 After three replacements, use a metering pump to inject liquid acetone into the preheater and heat it up to 60°C, then drop it into the reactor from the top of the reactor, and react in H2 2 Under the following conditions, the reaction pressure is 4MPa, the reactor inlet temperature is 90°C, and the liquid space velocity is 1.5h -1 , the acetone-hydrogen molar ratio is 1:8, and the reaction product is separated by light removal, weight removal and dehydration to obtain the product isopropanol, with a purity of 99.90%, a single-pass conversion rate of acetone of 98.8%, and the selectivity of isopropanol in terms of acetone is 99.4%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com