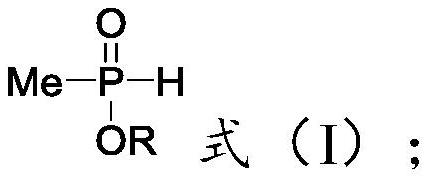Method for tubular continuous synthesis of (3-acetoxy-3-cyanopropyl)-methyl phosphinic acid alkyl ester
A technology of alkyl methyl phosphinate and alkyl methyl phosphinate, which is applied in the field of tubular continuous synthesis - alkyl methyl phosphinate, can solve the problem of high equipment cost and operating cost, process operation Complexity, complex operation and other issues, to achieve the effect of easy industrial control and continuous production, simple process steps and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
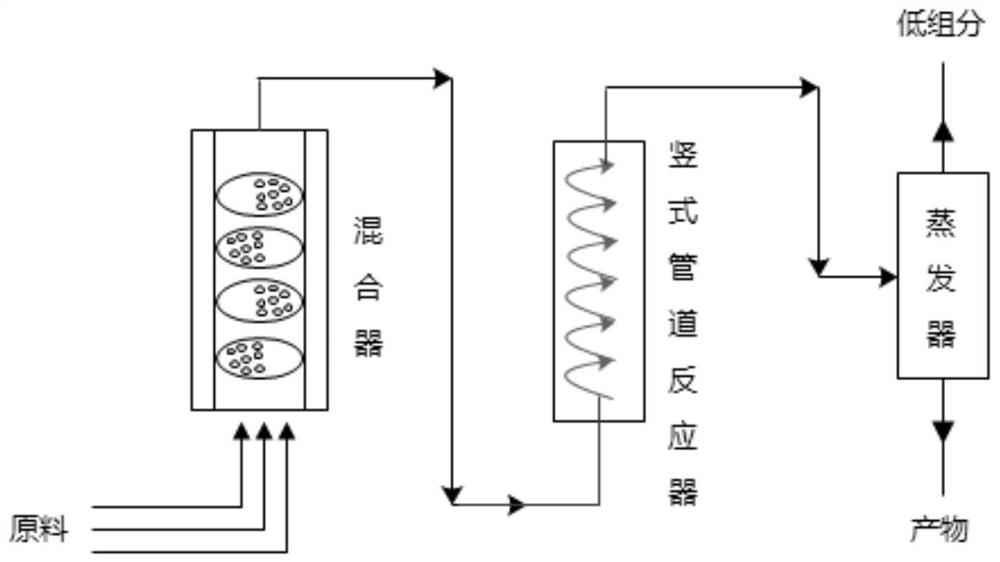
[0050] Adjust acetic acid-1-cyano-2-propenyl ester, isobutyl methyl phosphinate and tert-butyl peroxypivalate three materials to be metered into the mixer (5 orifice plates, each with 20 orifice), in which -1-cyano-2-propenyl acetate 4.12g / min, isobutyl methylphosphonite 13.31g / min, tert-butyl peroxypivalate 0.16g / min, mixed The temperature of the reactor is controlled at 0-5°C; after the mixed material is kept in the mixer for 0.1h, it directly enters the coil reactor, and the internal temperature of the reactor is controlled at 55-65°C, and the material stays in the coil reactor for 5 minutes. , directly transported to the thin-film evaporator, under the conditions of -0.090Mpa and 180°C, 8.39g / min (3-acetoxy-3-cyanopropyl)-methyl isobutyl phosphinate was obtained with a purity of 98.5% , yield 98.9%.
Embodiment 2
[0052] Adjust acetic acid-1-cyano-2-propenyl ester, monoethyl methylphosphonite and tert-butyl peroxyisooctanoate three materials into the mixer (7 orifice plates, each with 25 sections flow hole), wherein -1-cyano-2-propenyl acetate 4.12g / min, monoethyl methylphosphonite 26.62g / min, tert-butyl peroxyisooctanoate 0.33g / min, mixer temperature Control at 10-15°C; after the mixed material is kept in the mixer for 0.3h, it directly enters the annular tubular reactor, and the internal temperature of the reactor is controlled at 115-125°C, and the material stays in the annular tubular reactor for 10 minutes. , directly transported to the thin film evaporator, under the conditions of -0.098Mpa and 130°C, 8.31g / min (3-acetoxy-3-cyanopropyl)-methylphosphinic acid monoethyl ester was obtained with a purity of 98.7% , yield 98.2%.
Embodiment 3
[0054] Adjust acetic acid-1-cyano group-2-propenyl ester, methyl phosphinate mono-n-butyl ester and peroxyisooctanoic acid tert-butyl three materials to be metered into the mixer (10 orifice plates, each with 50 orifice), in which -1-cyano-2-propenyl acetate 4.12g / min, mono-n-butyl methylphosphonite 17.75g / min, tert-butyl peroxyisooctanoate 0.41g / min, mixed The temperature of the reactor is controlled at 5-10°C; after the mixed material is kept in the mixer for 1.0h, it directly enters the coil reactor, and the internal temperature of the reactor is controlled at 115-125°C, and the material stays in the coil reactor for 10 minutes. , directly transported to the evaporator, using molecular distillation, under the conditions of -0.099Mpa and 135°C, 8.28g / min (3-acetoxy-3-cyanopropyl)-methylphosphinic acid mono-n-butyl ester was obtained , the purity is 99.4%, and the yield is 98.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com