Side group functionalized polyamino acid block polymer, its preparation method and responsive reversible adhesive injectable hydrogel
A technology of block polymer and polyamino acid, which is applied in the field of responsive reversible adhesive injectable hydrogel and polyamino acid block polymer to achieve reversible change, reversible transformation, good biocompatibility and The effect of biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] The present invention also provides a method for preparing the above-mentioned side group functionalized polyamino acid block polymer, comprising the following steps:
[0062] The copolymer containing the A block and the C block is subjected to a click chemical reaction with the compound shown in formula (V) in an organic solvent to obtain a polyamino acid block polymer with side group functionalization; the A block is as follows: Shown in formula (I) or formula (II); the C block includes blocks shown in formula (III-A) and formula (III-D);
[0063]
[0064]
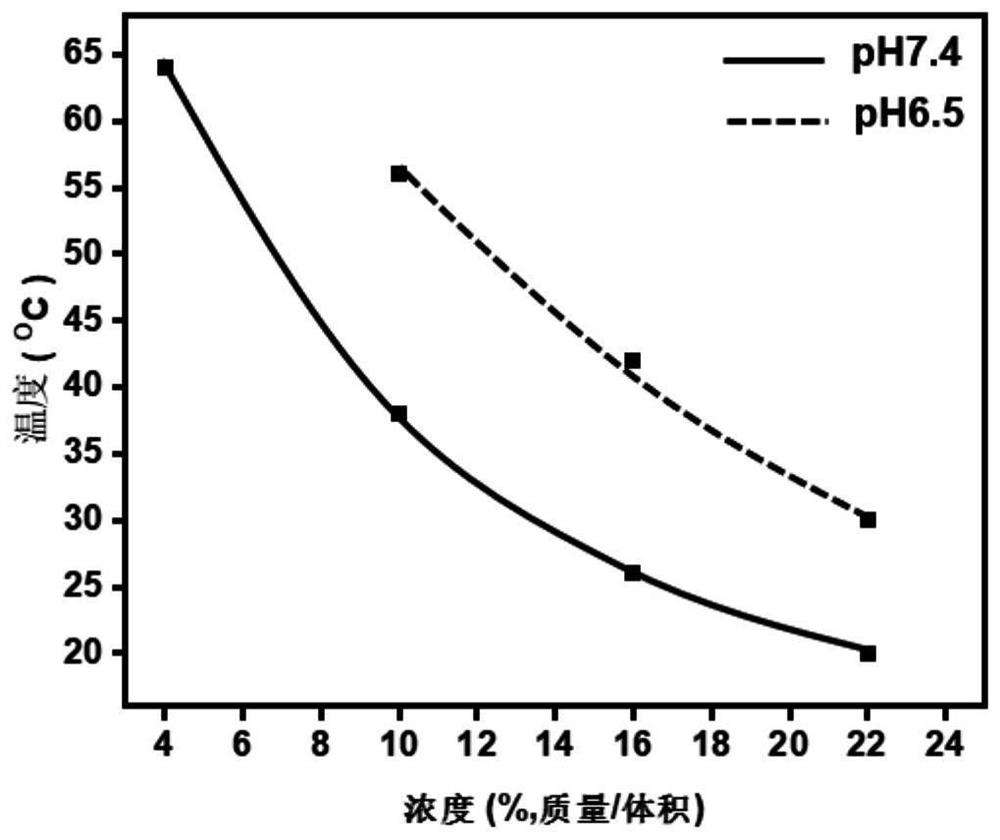
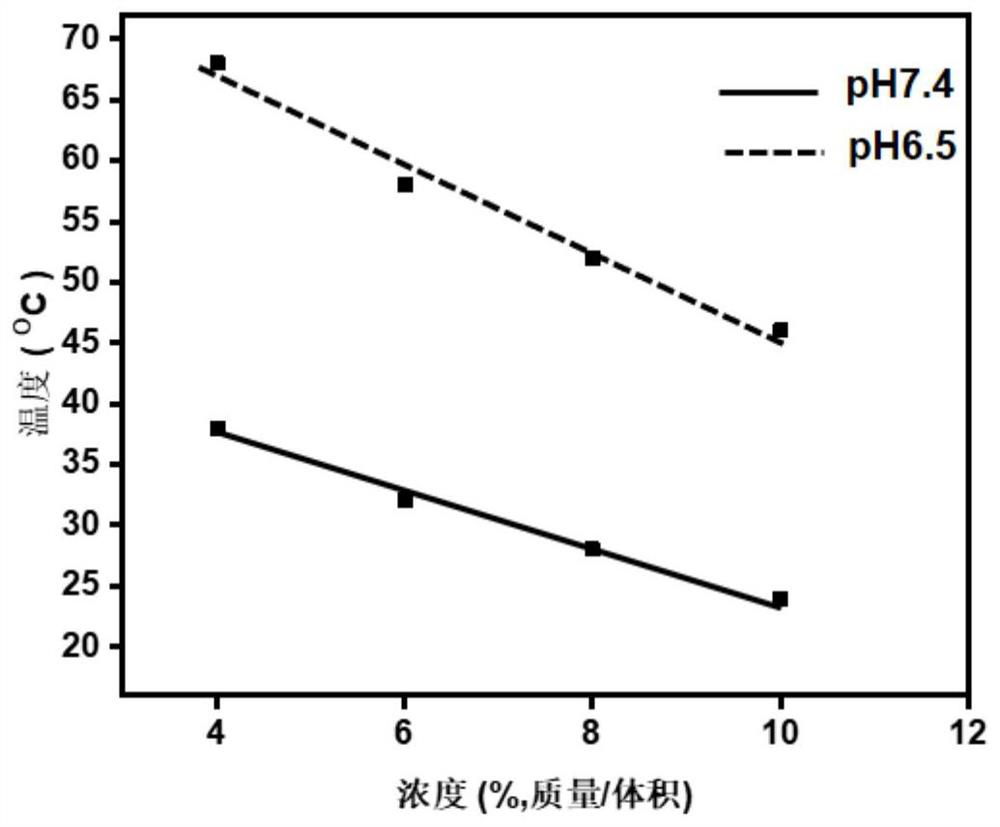
[0065] where x 1 、x 2 , y and p are degrees of polymerization, 10≤x 1 ≤113, 10≤x 2 ≤113, 0≤y≤15; 1≤p≤16, preferably 2≤p≤16, more preferably 4≤p≤16; in the examples provided by the present invention, p is specifically 6, 4, 8, 12, 15, 10, 5, 12 or 16; R 1 is C1~C5 alkyl; R 2 It is a C2-C5 terminal alkynyl group; b is an integer of 1-5, and c is an integer of 0-5. the x 1 、x 2 , y, R 1 , R 2 , b and...
Embodiment 1
[0089] Add 10g of 4-hydroxyethyl-1-methyl-piperazine and 10ml of thionyl chloride into 200ml of ultra-dry chloroform, reflux at 50°C for 12h, remove the solvent by rotary evaporation, dissolve in methanol, settle in ether, filter and Drying affords 4-chloroethyl-1-methyl-piperazine.
[0090] 13g of 4-chloroethyl-1-methyl-piperazine and 6g of sodium azide were dissolved in water, refluxed at 80°C for 24h, cooled to room temperature, extracted with ether, dried over anhydrous magnesium sulfate and rotary evaporated to obtain 4- Azidoethyl-1-methyl-piperazine.
Embodiment 2
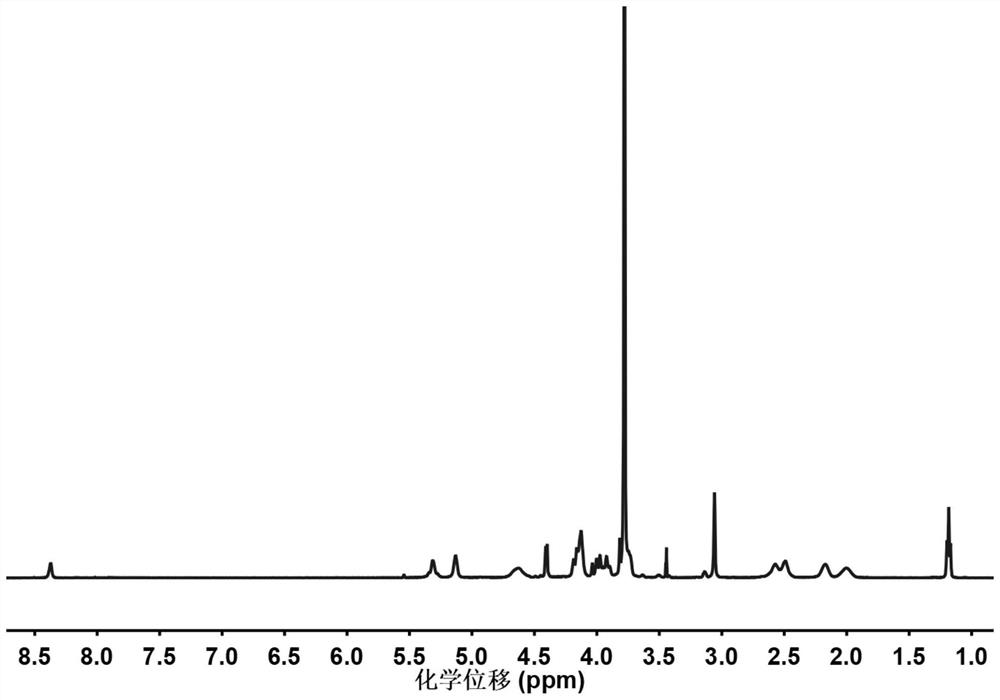
[0092] Add 2 g of aminated polyethylene glycol monomethyl ether with a number-average molecular weight of 2000 and 60 mL of anhydrous toluene to the dry reaction flask, and remove water by azeotroping at 130 ° C for 2 h, then vacuum the remaining toluene to dryness; The resulting solid was dissolved in 20 mL of dry N,N-dimethylformamide to obtain a first solution; 0.402 g of γ-ethyl-L-glutamate-N-carboxylate and 1.266 g The γ-propynyl-L-glutamate-N-carboxylate anhydride was dissolved in 40 mL of dry N,N-dimethylformamide to obtain the second solution; in a nitrogen atmosphere, the first solution Mix with the second solution, stir and react at room temperature under nitrogen protection for 72 hours; after the reaction, drain N,N-dimethylformamide under reduced pressure, then dissolve the obtained solid in chloroform, and then settle with ether , suction filtration, and drying to obtain polyethylene glycol monomethyl ether-poly(γ-ethyl-L-glutamate-c-γ-propynyl-L-glutamate) block...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com