Method for synthesizing L-alanine by catalyzing maleic acid through double-enzyme coupled whole cells
A technology of maleic acid and alanine, applied in the field of bioengineering, can solve the problems of long time consumption, high cost, complex fermentation process, etc., and achieve the effect of simple production method, high production efficiency and high output
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
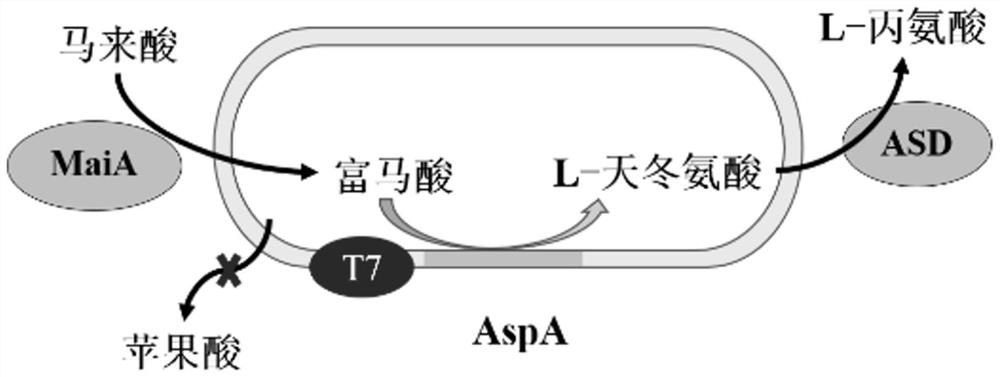
[0046] Example 1: Selection of L-aspartate β decarboxylase
[0047] Adjust the L-aspartate β decarboxylase to L-aspartate β decarboxylase from other 3 sources, respectively: ArASD (hereinafter referred to as ASD-1) of GenBank:AP019740.1, nucleic acid sequence such as SEQ ID NO .4 encoding L-aspartate β decarboxylase Pd21192ASD (hereinafter referred to as ASD-2), GenBank: WP_016451742.1 pET28a-Pd19121ASD (hereinafter referred to as ASD-3);
[0048] The recombinant strains E.coli BL21(DE3) / pET28a-ASD-1, E.coli BL21(DE3) / pET28a-ASD-2 and E.coli BL21(DE3) / pET28a-ASD-3 were synthesized by Jinweizhi Company and stored in In the preservation tube, the prepared single colony was inoculated in 5 mL of LB liquid medium with an antibiotic concentration of 50 mg / mL, cultivated at 37 ° C and 200 rpm for 8 h, and prepared a seed liquid; then inoculated with 2% (v / v) Transfer the seed solution to a 250mL shake flask containing 50mL of 2YT medium, the concentration of antibiotic kana is 50mg...
Embodiment 2
[0052] Example 2: Construction of a recombinant vector coupled to express maleate cis-trans isomerase and L-aspartate β decarboxylase
[0053] Specific steps are as follows:
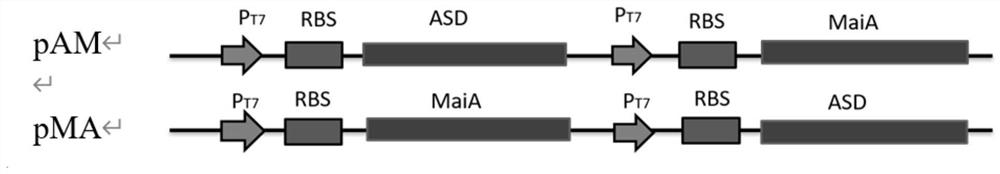
[0054] (1) According to the gene encoding maleate cis-trans isomerase shown in SEQ ID NO.2 and the gene encoding L-aspartic acid β decarboxylase shown in SEQ ID NO.4 according to the nucleic acid sequence and carrier, select the enzyme cutting site, and design primers, as shown in Table 1:
[0055] Table 1: Design of Enzyme Ligation Primers
[0056]
[0057] Note: the underlined mark is the restriction site
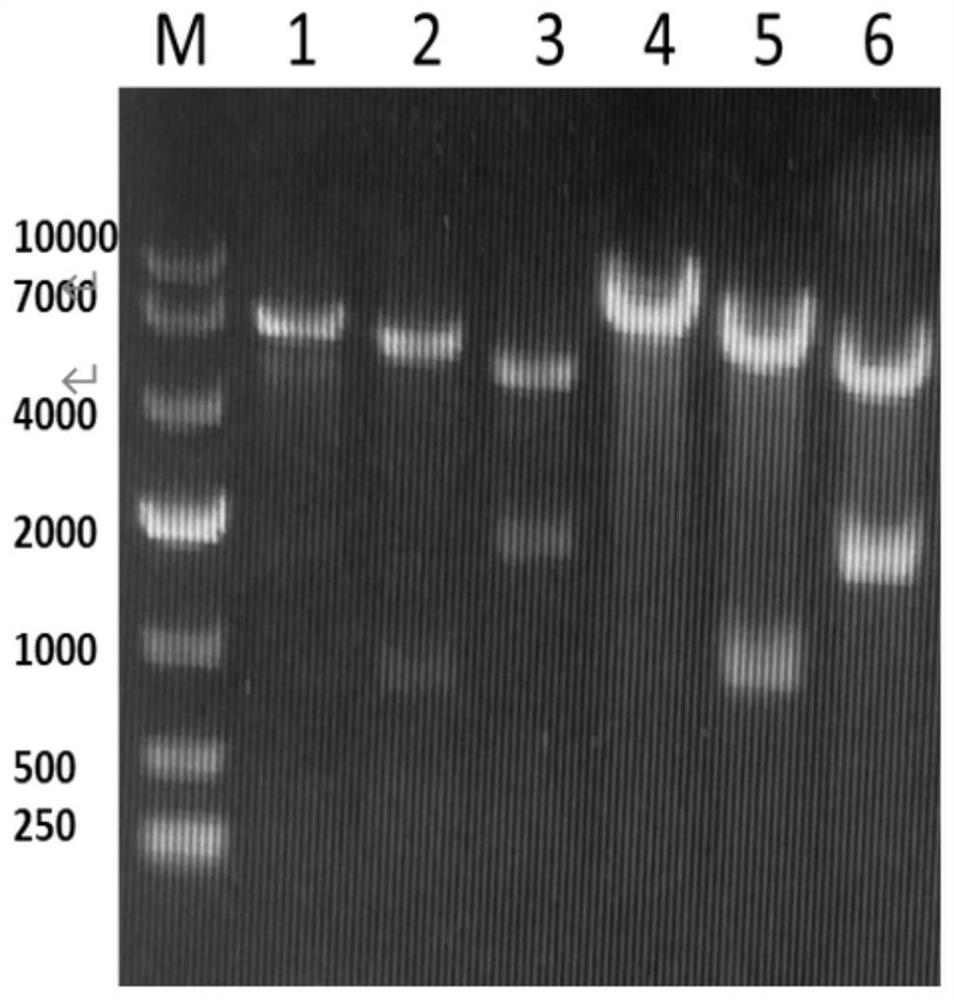
[0058] (2) chemically synthesized nucleic acid sequence as shown in SEQ ID NO.2 encoding maleate cis-trans isomerase gene MaiA and nucleic acid sequence as shown in SEQ ID NO.4 encoding L-aspartic acid β decarboxylase The gene ASD, using NdeⅠ, XhoⅠ, EcoRI, NotⅠ, BamHI, HindⅢ enzymes to the enzymes of the above genes and the plasmid vector pRSF Duet-1 Carry out double enzyme digestion for 4 ho...
Embodiment 3
[0061] Example 3: Construction of recombinant strains coupled to express maleate cis-trans isomerase and L-aspartate β decarboxylase and expression of enzymes
[0062] Specific steps are as follows:
[0063] (1) The recombinant plasmid pRSF prepared in Example 1 Duet-l -ASD-MaiA and pRSF Duet-l -MaiA-ASD was transformed into the expression host E.coli BL21(DE3)△fumAC competent cells, and then spread on the LB solid medium resistant to carrier kana, cultured at 37°C for 12h, and prepared E. .coli BL21(DE3)△fumAC / pRSF Duet-l -ASD-MaiA and E.coli BL21(DE3)△fumAC / pRSF Duet-l -MaiA-ASD.
[0064] (2) The single colony prepared in the picking step (1) was inoculated into 5 mL of LB medium with an antibiotic concentration of 50 mg / mL, cultured at 37 °C and 200 rpm for 8 hours, and then inoculated with 2% (v / v) The amount was transferred to a 250mL shake flask containing 50mL of 2YT medium, the concentration of antibiotic kana was 50mg / mL, and cultured to OD at 37°C and 200rpm 60...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com