Preparation method for green synthesis of N-acetyl-5-methoxytryptamine
A methoxytryptamine and green synthesis technology, which is applied in the preparation of organic compounds, carboxylic acid nitrile preparation, chemical instruments and methods, etc., can solve the problems of high energy consumption and high product loss, and achieve simplified processes, improved yields, Realize the effect of green synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
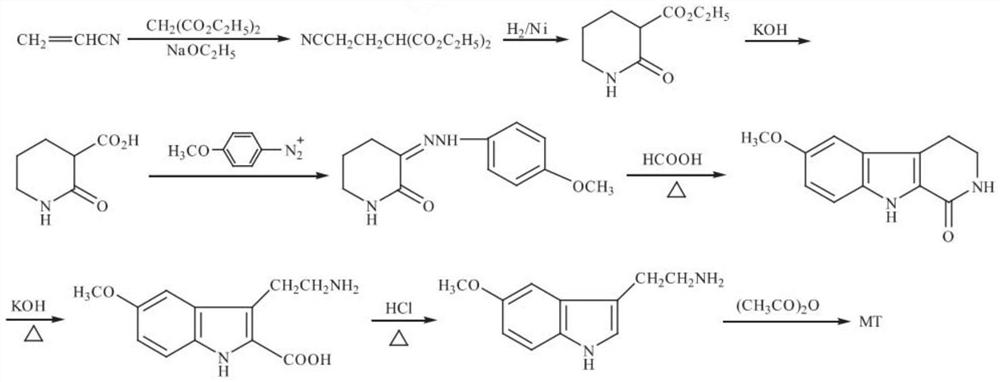
[0041] The present embodiment is a green preparation method for synthesizing N-acetyl-5-methoxytryptamine, the steps of which are as follows:
[0042] (1) Addition reaction
[0043]At 20°C, slowly add 15g of the basic catalyst sodium methoxide into 600g of diethyl malonate, and dropwise add 198g of acrylonitrile, and stir the reaction at 60°C for 3 hours. 30% sulfuric acid to neutralize to a pH value of 7, and then add 1600ml of water according to the volume ratio of the reaction solution: water = 1:2 for extraction; after extraction, the reaction solution is dehydrated by adding a dehydrating agent, anhydrous sodium sulfate, to a moisture content of 0.12% And obtain 800ml intermediate 1 reaction solution; Dehydrating agent is applied mechanically after drying, and waste water goes to biochemical system to process;
[0044] (2) Hydrogenation reaction
[0045] Transfer the above-mentioned 800ml intermediate 1 reaction liquid into the hydrogenation kettle, add 2400ml solvent m...
Embodiment 2
[0057] The present embodiment is a green preparation method for synthesizing N-acetyl-5-methoxytryptamine, the steps of which are as follows:
[0058] (1) Addition reaction
[0059] Slowly add 20g of basic catalyst sodium hydroxide at 30°C to 600g of diethyl malonate, and dropwise add 198g of acrylonitrile, and stir the reaction at 50°C for 2 hours. After the reaction is completed, add Acetic acid was neutralized to a pH value of 7, and then extracted three times according to the volume ratio of the reaction solution: water = 1:3 plus 2400ml of water; after extraction, the reaction solution was dehydrated by adding a dehydrating agent anhydrous magnesium sulfate until the water content was 0.1% to obtain 790ml of intermediate Body 1 reaction solution; the dehydrating agent is dried and applied mechanically, and the waste water is processed in the biochemical system;
[0060] (2) Hydrogenation reaction
[0061] Transfer the above-mentioned 790ml intermediate 1 reaction liquid...
Embodiment 3
[0073] The present embodiment is a green preparation method for synthesizing N-acetyl-5-methoxytryptamine, the steps of which are as follows:
[0074] (1) Addition reaction
[0075] At 30°C, slowly add 13g of basic catalyst triethylamine into 600g of diethyl malonate, and dropwise add 198g of acrylonitrile, and stir the reaction at 55°C for 3 hours. After the reaction is completed, add The concentration is 15% hydrochloric acid to neutralize to a pH value of 7, and then extract five times according to the volume ratio of the reaction solution: water = 1:5 plus 4000ml of water; after extraction, the reaction solution is dehydrated to water by adding a dehydrating agent anhydrous calcium chloride 0.15% to obtain 785ml intermediate 1 reaction solution; the dehydrating agent is dried and applied mechanically, and the waste water is removed to the biochemical system for treatment;
[0076] (2) Hydrogenation reaction
[0077] Transfer the above-mentioned 785ml intermediate 1 react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com