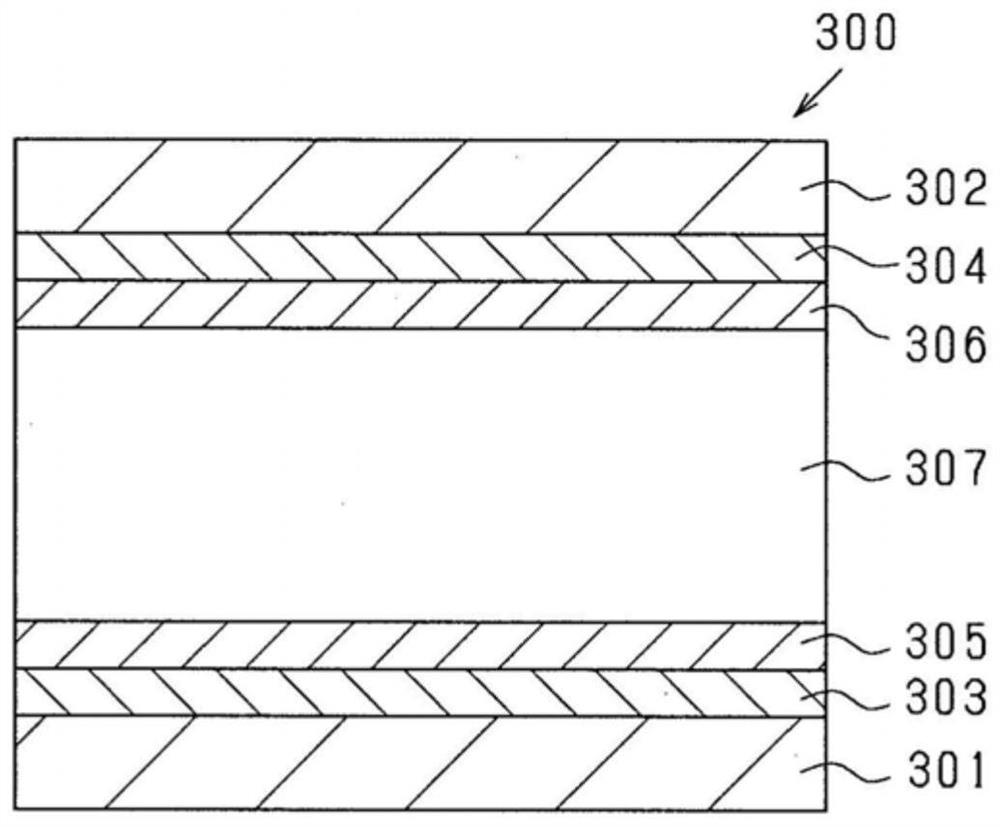
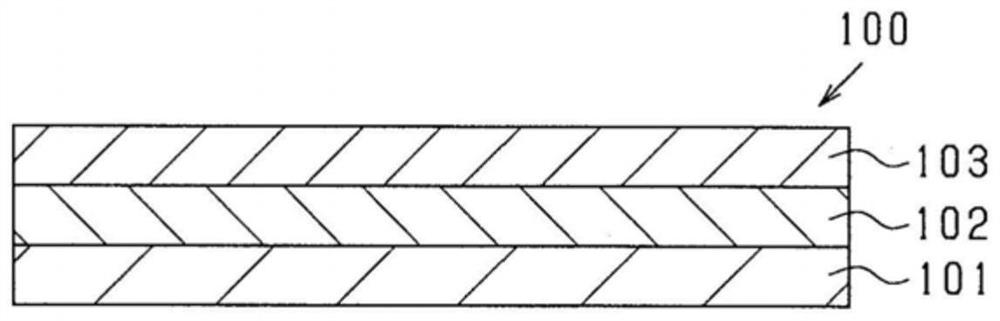
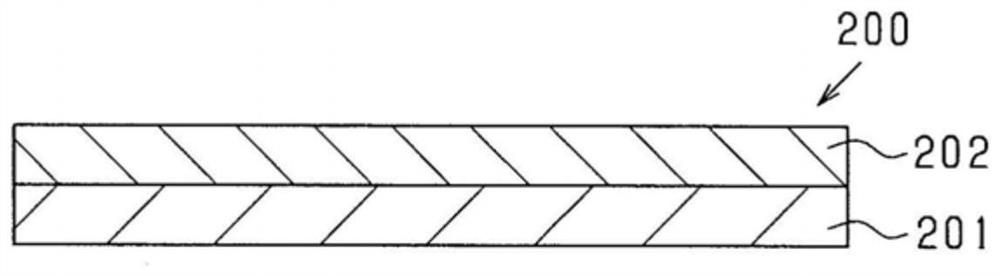
Composition and use therefor
A composition and compound technology, applied in coatings, instruments, liquid crystal materials, etc., can solve problems such as the difficulty in solubility of rigid rod-shaped polymers, and achieve low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0230] Hereinafter, although an Example demonstrates more concretely, this invention is not limited to these Examples.
[0231] The structures and abbreviations of the main compounds used in the following examples are as follows.
[0232] (tetracarboxylic dianhydride)
[0233] TA-1; pyromellitic dianhydride
[0234] TA-2; 1,4,5,8-naphthalene tetracarboxylic dianhydride
[0235] TA-3; 2,3,4,7-naphthalene tetracarboxylic dianhydride
[0236] TA-4; 4,4'-diphthalic dianhydride
[0237] [chemical 17]
[0238]
[0239] (diamine)
[0240] DA-1; 2,5-diaminobenzenesulfonic acid
[0241] DA-2; 4,4'-Diamino-2,2'-biphenyldisulfonic acid
[0242] DA-3; 4,4'-diaminostilbene-2,2'-disulfonic acid
[0243] [chemical 18]
[0244]
[0245] (basic monomer)
[0246] M-1; N,N-Dimethylaminopropylacrylamide
[0247] (polymerization initiator)
[0248] I-1; Azobis[2-(-imidazolin-2-yl)propane] disulfate dihydrate
[0249] I-2; 2-Hydroxy-4'-(2-hydroxyethoxy)-2-methylpropiophenone
[...
Synthetic example 1
[0254] (1) Synthesis of polymer
[0255] Put diamine (DA-1) (1.88g, 10.0mmol), m-cresol (30mL) and triethylamine (2.43g, 24.0mmol) in a three-necked flask equipped with a reflux tube, a thermometer, and a nitrogen gas introduction tube. Stirring was carried out at 80°C under nitrogen. After dissolving diamine, acid dianhydride (TA-1) (2.09g, 9.6mmol) and benzoic acid (1.71g, 14.0mmol) were added, and after stirring at 80 degreeC for 3 hours, it stirred at 180 degreeC for 12 hours. Accompanied by the imide ring-closing reaction, the polymer gradually precipitated, and the reaction mixture changed from a black-yellow solution to an orange-red slurry. After the reaction was completed, the reaction mixture was left to cool, and poured into acetone to solidify. The obtained coagulum was filtered, stirred and washed in acetone, then stirred and washed in isopropanol, and vacuum-dried at 120° C., thereby obtaining the repeating unit constituting the polymer comprising the following...
Embodiment 1
[0271] (1) Preparation of the composition
[0272] The polymer (PI-1) obtained in Synthesis Example 1 was dissolved in water, and ion-exchanged by a strongly acidic cation-exchange resin, thereby obtaining a pair of cations from Et 3 NH + was replaced by H + Aqueous solution of acidic polymer (hereinafter referred to as "acidic polymer (PI-1A")). Dialysis tube made of regenerated cellulose (cut-off molecular weight 3500, manufactured by Spectra / Por) The obtained aqueous solution was dialyzed at room temperature against distilled water to further refine it. After the dialyzed aqueous solution was concentrated under reduced pressure, an acidic functional group was added to the acidic polymer (PI-1A) to obtain 1 molar equivalent of the basic monomer (M-1), then add the polymerization initiator (I-1) and dissolve it. By using a filter with a pore size of 0.45 μm, the obtained aqueous solution is filtered to prepare an acidic polymer (PI-1A) and the composition (C-1) in which th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com